
APA Style
Mohamed Hussein. (2025). Epigenetic Insights into Bladder Cancer: The Role of Urine DNA Methylation Assays: Mini Review. GenoMed Connect, 2 (Article ID: 0015). https://doi.org/10.69709/GenomC.2025.145625MLA Style
Mohamed Hussein. "Epigenetic Insights into Bladder Cancer: The Role of Urine DNA Methylation Assays: Mini Review". GenoMed Connect, vol. 2, 2025, Article ID: 0015, https://doi.org/10.69709/GenomC.2025.145625.Chicago Style
Mohamed Hussein. 2025. "Epigenetic Insights into Bladder Cancer: The Role of Urine DNA Methylation Assays: Mini Review." GenoMed Connect 2 (2025): 0015. https://doi.org/10.69709/GenomC.2025.145625.
 ACCESS
Review Article
ACCESS
Review Article
Volume 2, Article ID: 2025.0015

Mohamed Hussein
dr.m.hussin@dmcg.edu
Received: 08 Apr 2025 Accepted: 02 Jun 2025 Available Online: 02 Jun 2025 Published: 24 Jun 2025
Bladder cancer remains a major global health challenge due to its high incidence, recurrence rates, and reliance on invasive diagnostic procedures. While conventional methods, such as cystoscopy and urine cytology, are considered standard, they often demonstrate limited sensitivity, particularly in detecting early-stage disease. Epigenetic alterations, particularly DNA methylation, are now recognized as critical in bladder cancer development and progression. Non-invasive urine-based DNA methylation assays have emerged as promising diagnostic and monitoring tools by detecting tumor-derived molecular changes in exfoliated DNA. Notably, hypermethylation of tumor suppressor gene promoters such as CDKN2A, RASSF1A, and DAPK, as well as hypomethylation in oncogene-associated regions like MYC and CCND1, have shown strong associations with bladder cancer presence and severity. Recent advancements in detection technologies, including methylation-specific PCR (MSP), droplet digital PCR (ddPCR), and Next-Generation Sequencing (NGS), have significantly improved sensitivity and specificity, enabling earlier diagnosis, better risk stratification, and the development of personalized management strategies. Clinical studies also point to the potential of novel markers like TWIST1, NID2, and mDMRTA2 in enhancing diagnostic accuracy. Despite these advances, broader clinical validation and standardized testing protocols are essential for integrating methylation assays into routine clinical practice. These assays represent a transformative shift toward non-invasive, accurate, and patient-centric bladder cancer care.
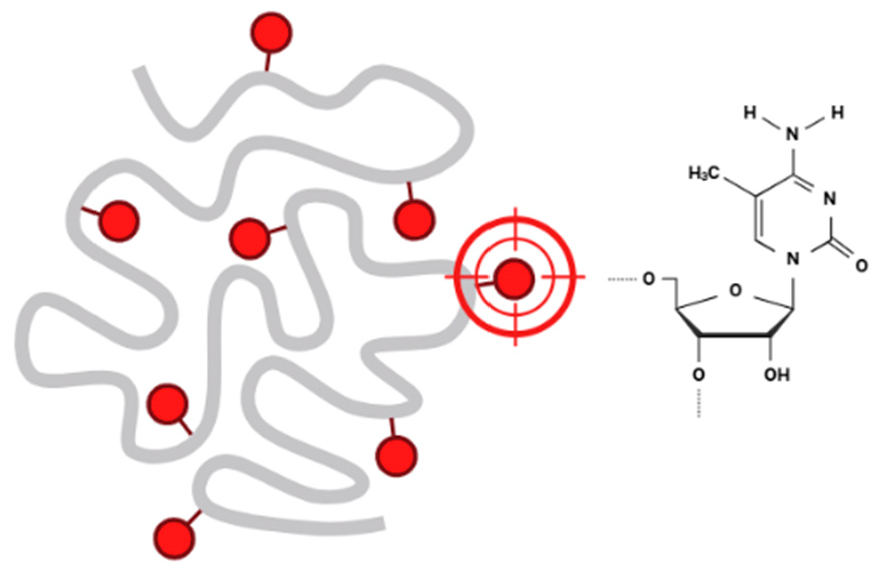
1.1. Bladder Cancer Bladder cancer is a significant global health concern due to its high incidence, substantial mortality, and the challenges it presents in diagnosis and treatment. It is a heterogeneous disease with varying clinical presentations and outcomes, requiring individualized management strategies. Men are affected about three to four times more often than women, likely due to differences in exposure to risk factors such as smoking and occupational carcinogens [1] (Figure 1). The median age at diagnosis is 73 years, making it a disease predominantly of the elderly. Bladder cancer accounts for an estimated 213,000 deaths annually worldwide. While non-muscle-invasive bladder cancer (NMIBC) has a lower mortality rate, muscle-invasive bladder cancer (MIBC) and metastatic cases contribute significantly to mortality [2,3]. 1.2. The Critical Role of Early Detection in Bladder Cancer Management Early detection of bladder cancer is pivotal for improving outcomes, as it enables timely interventions that significantly enhance survival rates, prevent disease progression, and reduce the burden of advanced treatments. Early-stage bladder cancer, particularly non-muscle-invasive bladder cancer (NMIBC), has a far better prognosis compared to advanced stages, with a 5-year survival rate of ~77% for localized disease, compared to ~30% for regional spread and <10% for distant metastases. Early identification of NMIBC enables effective management with intravesical therapies, such as Bacillus Calmette-Guérin (BCG), preventing progression to muscle-invasive bladder cancer (MIBC), which is more aggressive and requires radical treatments [4,5,6]. Early diagnosis broadens treatment options, favoring minimally invasive approaches like transurethral resection of bladder tumors (TURBT) and bladder-preserving therapies, while reducing the need for radical cystectomy and systemic chemotherapy, thereby preserving bladder function and quality of life. It also facilitates better risk stratification, allowing high-risk patients to be closely monitored for recurrence or progression using advanced non-invasive diagnostic tools, such as urine-based DNA methylation assays and NMP22 tests, which improve patient compliance and screening feasibility. Furthermore, early detection reduces the costs associated with lifelong surveillance and advanced disease management, helps prevent metastasis through timely systemic and targeted therapies, and ultimately improves survival outcomes while lessening the physical, emotional, and financial burdens on patients [7,8,9,10]. 1.3. The Role of DNA Methylation in Epigenetic Regulation and Development DNA methylation is a fundamental epigenetic mechanism involving the addition of a methyl group to the fifth carbon of the cytosine ring, primarily within CpG dinucleotides (Figure 2). This reaction is catalyzed by DNA methyltransferases (DNMTs), including DNMT1 (which maintains methylation during DNA replication) and DNMT3A/3B (which establish new methylation patterns). Methylation in promoter regions typically represses gene transcription by recruiting methyl-CpG-binding domain proteins (MBDs), which compact chromatin and restrict transcriptional access. This process is vital for regulating gene expression, silencing repetitive sequences, and maintaining genomic stability. However, when disrupted, it can contribute to the development of different diseases, including cancer [11,12,13,14]. DNA methylation plays a vital role in gene regulation by influencing transcriptional activity, genomic stability, and cellular processes. Methylation in gene promoter regions is closely linked to transcriptional repression, as methylated promoters recruit methyl-CpG-binding domain proteins (MBDs) that form complexes with histone-modifying enzymes to compact chromatin, restricting access to the transcriptional machinery. This mechanism is critical during development, such as silencing pluripotency genes in differentiated cells. Additionally, DNA methylation helps to maintain genomic stability by silencing repetitive sequences and transposable elements, preventing inappropriate recombination and genomic instability [15]. It contributes to X-chromosome inactivation in females, equalizing gene dosage with males, and plays a role in genomic imprinting by regulating parent-specific expression of certain genes essential for normal growth and development. However, aberrant DNA methylation is implicated in various diseases, particularly cancer. Hypermethylation of tumor suppressor gene promoters, such as p16 or MLH1, leads to gene silencing and facilitates uncontrolled cell proliferation. Conversely, global hypomethylation, a hallmark of many cancers, activates oncogenes and transposable elements, increasing genomic instability. Thus, DNA methylation is a dynamic process that maintains cellular homeostasis but, when disrupted, contributes to disease pathogenesis [16,17,18]. 1.4. DNA Methylation and Bladder Cancer: A Dual Role in Genomic Stability and Tumorigenesis In bladder cancer, DNA methylation patterns become dysregulated, contributing to tumorigenesis and disease progression. Two major types of alterations are commonly observed: global hypomethylation and locus-specific methylation changes [19]. 1.4.1. Global Hypomethylation Global hypomethylation involves the loss of methylation in repetitive sequences, transposable elements, and intergenic regions. It is often observed in later stages of bladder cancer. Genomic Instability: Hypomethylation activates transposable elements (e.g., LINE-1) and leads to chromosomal instability. Oncogene Activation: Hypomethylation in promoter regions of oncogenes results in overexpression, promoting tumor growth and progression. Hypomethylation of LINE-1 sequences is a potential biomarker for bladder cancer prognosis. Hypomethylation in non-coding regulatory regions can alter enhancer activity, leading to abnormal expression of nearby oncogenes or loss of regulatory control [20]. 1.4.2. Targeted Methylation Alterations of Tumor Suppressors and Oncogenes in Bladder Cancer Locus-specific methylation changes involve hypermethylation or hypomethylation of specific genes or genomic regions. These changes are often more directly associated with bladder cancer development and progression. Hypermethylation occurs predominantly in CpG islands within the promoter regions of tumor suppressor genes. It leads to transcriptional silencing of these genes. Promoter hypermethylation of genes like CDKN2A and RASSF1A is a promising diagnostic and prognostic marker. Localized hypomethylation can occur in the promoter or enhancer regions of oncogenes, resulting in the overexpression of genes that drive tumorigenesis. Oncogenes such as MYC and CCND1 can exhibit hypomethylation in their regulatory regions, leading to their upregulation. Hypomethylation in cancer-testis antigens (e.g., MAGE-A1) is frequently observed in bladder cancer [21,22]. 1.5. Unlocking the Diagnostic Potential of Urine in Bladder Cancer Urine serves as an excellent diagnostic medium for bladder cancer due to its accessibility and the non-invasive nature of its collection, making it ideal for repeated sampling and monitoring. As a direct contact medium with the bladder, urine contains a wealth of molecular information shed by tumor cells, allowing for the detection of a variety of biomarkers. These include DNA (e.g., mutations, methylation patterns), RNA (e.g., microRNAs, mRNAs), proteins (e.g., NMP22, cytokeratins), and metabolites, all of which can provide valuable insights into tumor biology and progression. The ease of urine collection reduces patient discomfort compared to invasive procedures like cystoscopy, while its composition reflects dynamic changes in the tumor microenvironment, offering opportunities for early detection, risk stratification, and monitoring of treatment responses. These attributes position urine-based diagnostics as a powerful tool for improving the non-invasive management of bladder cancer [23]. Urine DNA methylation assays have emerged as a promising tool for the non-invasive diagnosis and monitoring of bladder cancer. These assays detect abnormal methylation patterns in cell-free DNA or exfoliated tumor cells present in the urine, offering insights into tumor biology and epigenetic alterations associated with cancer. Aberrant DNA methylation, such as hypermethylation of tumor suppressor gene promoters (e.g., CDKN2A, RASSF1A, DAPK), is a hallmark of bladder cancer and plays a critical role in tumor progression and recurrence. The high sensitivity and specificity of methylation assays make them particularly valuable for detecting bladder cancer at early stages, even before visible tumors are detected by imaging or cystoscopy. Additionally, these assays are ideal for monitoring patients during follow-up to detect residual disease or recurrence, reducing the need for invasive procedures. Advances in technologies such as methylation-specific PCR (MSP), droplet digital PCR (ddPCR), and next-generation sequencing (NGS) have enhanced the reliability and scalability of these tests. Urine DNA methylation assays hold immense potential not only for improving early detection but also for risk stratification and guiding personalized treatment strategies, making them a key innovation in bladder cancer management [24,25]. Clinical trials have demonstrated the utility of urine-based DNA methylation assays for various clinical objectives in bladder cancer management, including diagnosis, monitoring, and biomarker discovery. For early diagnosis, techniques with high sensitivity, such as quantitative methylation-specific PCR (qMSP) and droplet digital PCR (ddPCR), have been evaluated for their ability to detect low-abundance methylated DNA associated with bladder tumors. For instance, a trial investigating the methylation of CDKN2A and RASSF1A in urine samples reported high sensitivity and specificity for distinguishing bladder cancer patients from healthy controls, even in early-stage disease. These findings highlight the potential of qMSP and ddPCR to facilitate early detection, reducing reliance on invasive cystoscopy and improving patient compliance [25,26]. In the context of disease monitoring, precision techniques like ddPCR and targeted next-generation sequencing (NGS) have shown promise in detecting residual disease and predicting recurrence. A multicenter trial utilizing ddPCR to monitor RASSF10 and APC methylation in urine demonstrated that methylation levels correlate strongly with recurrence risk, providing a non-invasive method for long-term surveillance. Such approaches enable timely intervention, improving outcomes while reducing the need for frequent invasive procedures. Additionally, studies combining ddPCR with other urine biomarkers, such as protein or RNA signatures, have enhanced the predictive power of these assays for recurrence detection. For biomarker discovery, whole-genome or targeted NGS platforms have been pivotal in identifying novel methylation markers for bladder cancer. Trials utilizing (NGS) to profile urine samples have identified several methylation candidates, such as ZIC4 and GATA4, which can distinguish between different subtypes and stages of bladder cancer. These biomarkers are being validated in ongoing clinical trials for their diagnostic, prognostic, and predictive utility. Furthermore, NGS-based studies have revealed methylation patterns specific to high-risk bladder cancer, aiding in patient stratification and the development of personalized treatment strategies [23,24]. 1.6. Comparative Analysis of Urine DNA Methylation Assays and Other Bladder Cancer Biomarkers Urine-based DNA methylation assays have emerged as highly promising tools in bladder cancer diagnostics, specifically when evaluated alongside established and emerging urinary biomarkers such as cytology, NMP22, UroVysion FISH, and multiplex platforms like Cxbladder. While urine cytology is highly specific (>90%), its low sensitivity, particularly for low-grade tumors, limits its use as a standalone diagnostic [27]. NMP22 provides moderate sensitivity (50–70%) but lower specificity and can yield false positives in benign conditions, whereas UroVysion FISH offers improved accuracy for high-grade tumors with 60–80% sensitivity and 70–85% specificity. Cxbladder, which measures multi-gene mRNA signatures, shows high negative predictive value, making it useful for ruling out malignancy [28]. By comparison, DNA methylation assays targeting genes like CDKN2A, RASSF1A, and TWIST1 demonstrate consistently high sensitivity (70–90%) and specificity (85–95%) even for early-stage disease [29]. For initial diagnosis in patients with hematuria, combining methylation markers with traditional tests like cytology or NMP22 enhances diagnostic sensitivity without compromising specificity. During follow-up care, these assays also reduce reliance on invasive cystoscopy, with studies confirming their utility in surveillance settings [29,30]. There is also growing interest in combining DNA methylation assays with other biomarker types such as proteins (e.g., NMP22) and RNA signatures (e.g., Cxbladder) to boost accuracy and reduce diagnostic uncertainty. These approaches are particularly beneficial for high-risk patients or when test results are inconclusive, helping to improve clinical decision-making and avoid missed diagnoses or unnecessary procedures [31,32]. While molecular tools like UroVysion and Cxbladder offer enhanced performance, they come with higher costs and require specialized infrastructure, limiting their accessibility. In contrast, methylation assays using techniques such as qMSP and ddPCR offer strong diagnostic accuracy with better scalability and cost-efficiency. These assays can be centralized, streamlining laboratory processes and enabling integration with other molecular diagnostics, an advantage for clinics seeking accurate, practical, and affordable bladder cancer testing options [24,33,34]. 1.7. CDKN2A, RASSF1A, and Beyond: A Clinical Perspective on Urinary DNA Methylation Testing in Bladder Cancer Several clinical studies have investigated the potential of urinary DNA methylation biomarkers such as CDKN2A and RASSF1A in the diagnosis of bladder cancer, each contributing valuable insights into assay performance and clinical applicability. A large multicenter study evaluated a methylation-based urine test in over 1000 patients with hematuria, demonstrating high sensitivity (89.2%) and specificity (87.8%), notably outperforming traditional methods like urine cytology and NMP22 [35]. Similarly, a study validated a qMSP-based panel including GHSR and MAL, achieving 80% sensitivity and 93% specificity in urine samples [36]. Earlier research reviewed the broader landscape of DNA methylation assays, comparing detection technologies such as ddPCR and NGS, and highlighting challenges related to assay standardization and variability in DNA sources [37]. Supporting these findings, another study reported a correlation between CDKN2A and RASSF1A promoter hypermethylation and tumor grade in urothelial carcinoma, reinforcing their prognostic relevance [38]. More recently, a novel marker, mDMRTA2, was introduced and validated through a urine-based assay with strong diagnostic accuracy [39]. Collectively, these studies underscore the growing evidence for urinary methylation biomarkers while emphasizing the need for standardized methodologies, larger cohorts, and real-world validation to support clinical adoption. 1.8. Multi-Omics and Machine Learning: Unlocking Precision Oncology in Bladder Cancer Recent progress in cancer systems biology has underscored the growing impact of multi-omics integration in refining prognostic models for bladder cancer. By merging DNA methylation data with other molecular layers such as transcriptomics and genomic alterations, researchers are gaining a more complete understanding of tumor dynamics. For example, one study used a machine learning–based multi-omics approach to investigate mitotic catastrophe heterogeneity in bladder cancer, identifying distinct molecular subtypes and the gene ANLN as a significant prognostic marker, demonstrating how integrative strategies can uncover meaningful biological patterns [40]. Another study combined RNA-seq, miRNA-seq, methylation profiles, and number variations using a transfer learning-based Cox model, resulting in improved prognostic accuracy across various datasets [41]. Integrating multi-omics data with clinical treatment information has revealed gene combinations associated with both treatment response and patient outcomes, thereby highlighting novel therapeutic opportunities [42]. This approach isn’t limited to bladder cancer; for instance, a study in endometrial carcinoma identified a novel methylation-based prognostic signature by linking methylation with gene expression data [43]. Within bladder cancer specifically, large-scale methylation analyses have identified key prognostic biomarkers [44], and early-stage genomic studies have provided insights into molecular subtypes and potential treatment targets [45]. In parallel, the incorporation of artificial intelligence (AI) and machine learning (ML) into multi-omics analysis has accelerated advances in cancer diagnostics and risk prediction. Deep learning methods have been particularly successful at integrating complex datasets, with a benchmark study revealing that models like moGAT are strong performers in classification, while efmmdVAE and efVAE show strength in clustering tasks [46]. These findings suggest that selecting the right model depends heavily on the research objective and data structure. Graph-based approaches are also gaining ground—models like LASSO-MOGAT have achieved high accuracy by integrating mRNA, miRNA, and methylation data through Graph Attention Networks and LASSO-based feature selection [47]. Together, these developments highlight the promise of AI-driven, multi-omics strategies in advancing precision medicine and improving patient outcomes in oncology.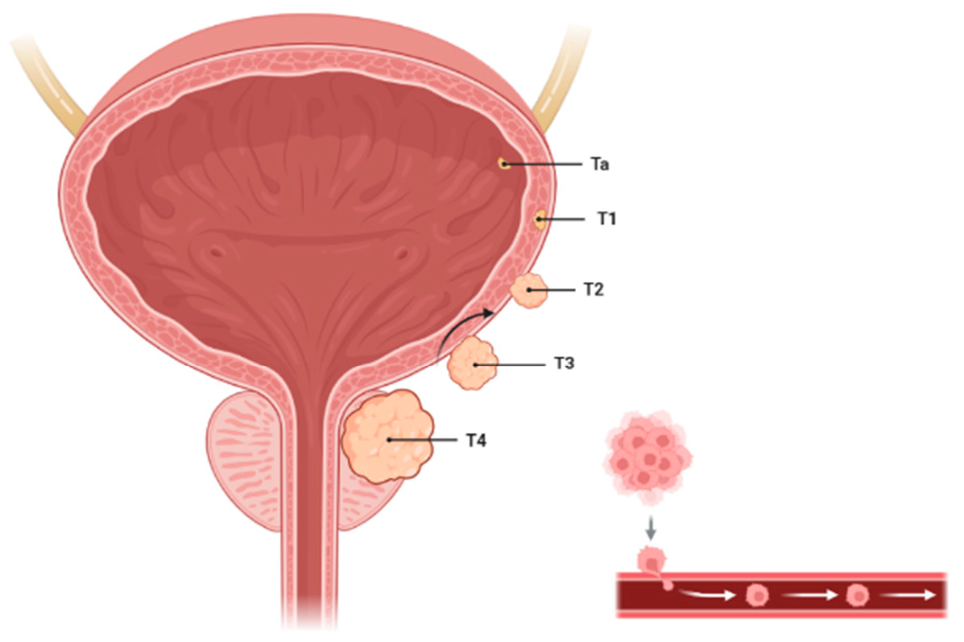
An extensive literature review was carried out using PubMed, Scopus, and Web of Science to identify relevant publications from 2015 to 2025 concerning urine-based DNA methylation assays for bladder cancer. The search utilized keywords such as “bladder cancer,” “urine DNA methylation,” “non-invasive biomarkers,” “epigenetic assays,” “qMSP,” “ddPCR,” and “NGS.” Studies were included if they were original research articles or clinical trials involving human participants, focused on the diagnostic or prognostic use of DNA methylation markers in urine, and reported quantitative performance metrics such as sensitivity, specificity, or area under the curve (AUC). Exclusion criteria encompassed studies using non-urine specimens (e.g., tissue or blood), in vitro or animal-only studies, and non-primary research such as reviews. Studies lacking quantitative outcomes related to methylation biomarkers or focusing exclusively on treatment response without diagnostic relevance were also excluded. The initial search identified 299 articles. After screening titles and abstracts, 75 full-text articles were evaluated, resulting in 41 studies that met all inclusion criteria and were analyzed in this review.
Bladder cancer continues to pose a significant global health challenge due to its high prevalence, associated mortality, and dependence on invasive diagnostic techniques. Advances in the understanding of epigenetic mechanisms, particularly DNA methylation, have introduced innovative approaches for diagnosis and monitoring. DNA methylation plays a central role in bladder cancer progression, with global hypomethylation leading to genomic instability and locus-specific methylation changes either silencing tumor suppressor genes or activating oncogenes. These discoveries have facilitated the development of urine-based DNA methylation assays, offering a non-invasive, patient-friendly alternative for early detection, risk assessment, and disease surveillance. Techniques such as methylation-specific PCR (MSP), droplet digital PCR (ddPCR), and next-generation sequencing (NGS) demonstrate high sensitivity and specificity, making them indispensable in clinical and research settings. Clinical studies have highlighted the effectiveness of these assays in identifying early-stage bladder cancer, tracking residual disease, and discovering novel biomarkers for personalized treatment approaches. By minimizing the need for invasive procedures like cystoscopy, urine-based assays improve patient compliance and support more feasible long-term monitoring. Moreover, the incorporation of methylation markers into routine clinical practice holds great potential to revolutionize bladder cancer management by enabling individualized treatment strategies and improving outcomes. Nevertheless, additional research and extensive clinical validation are needed to confirm the scalability, reliability, and cost-effectiveness of these methods in everyday healthcare applications. As the field evolves, urine DNA methylation assays are poised to reshape the future of bladder cancer diagnosis and care, providing a non-invasive, precise, and patient-centered approach.
| BCG | Bacillus Calmette-Guérin |
| CpG | Cytosine-phosphate-Guanine |
| ddPCR | Droplet Digital Polymerase Chain Reaction |
| DNA | Deoxyribonucleic Acid |
| DNMT | DNA Methyltransferase |
| DNMT1 | DNA Methyltransferase 1 |
| DNMT3A | DNA Methyltransferase 3 Alpha |
| DNMT3B | DNA Methyltransferase 3 Beta |
| MBD | Methyl-CpG Binding Domain |
| MIBC | Muscle-Invasive Bladder Cancer |
| MSP | Methylation-Specific Polymerase Chain Reaction |
| NGS | Next-Generation Sequencing |
| NMIBC | Non-Muscle-Invasive Bladder Cancer |
| NMP22 | Nuclear Matrix Protein 22 |
| PCR | Polymerase Chain Reaction |
| qMSP | Quantitative Methylation-Specific PCR |
| RNA | Ribonucleic Acid |
| TURBT | Transurethral Resection of Bladder Tumor |
The author conceptualized the study and conducted the liter-ature review. He was responsible for drafting the manuscript, interpreting the data, and revising it critically for important intellectual content. All sections of the manuscript were written and reviewed solely by the author. The author ap-proved the final version of the manuscript and agrees to be accountable for all aspects of the work.
The author declares no conflicts of interest.
This research received no external funding.
The author would like to thank Dubai Medical College for girls for their support during the study. The author used BioRender software for the creation of figures.
[1] Dyrskjøt, L.; Hansel, D.E.; Efstathiou, J.A.; Knowles, M.A.; Galsky, M.D.; Teoh, J.; Theodorescu, D. Bladder Cancer. Nat. Rev. Dis. Primers 2023, 9, 58. [CrossRef] [PubMed]
[2] Lidagoster, S.; Ben-David, R.; De Leon, B.; Sfakianos, J.P. BCG and Alternative Therapies to BCG Therapy for Non-Muscle-Invasive Bladder Cancer. Curr. Oncol. 2024, 31, 1063–1078. [CrossRef]
[3] Xu, Y.; Luo, C.; Wang, J.; Chen, L.; Chen, J.; Chen, T.; Zeng, Q. Application of Nanotechnology in the Diagnosis and Treatment of Bladder Cancer. J. Nanobiotechnol. 2021, 19, 1–18. [CrossRef]
[4] Siracusano, S.; Rizzetto, R.; Porcaro, A.B. Bladder Cancer Genomics. Urol. J. 2020, 87, 49–56. [CrossRef] [PubMed]
[5] Dobruch, J.; Oszczudłowski, M. Bladder Cancer: Current Challenges and Future Directions. Medicina 2021, 57. [CrossRef]
[6] Hamad, J.; McCloskey, H.; Milowsky, M.I.; Royce, T.; Smith, A. Bladder Preservation in Muscle-Invasive Bladder Cancer: A Comprehensive Review. Int. Braz. J. Urol. 2020, 46, 169–184. [CrossRef]
[7] Holzbeierlein, J.M.; Bixler, B.R.; Buckley, D.I.; Chang, S.S.; Holmes, R.; James, A.C.; Kirkby, E.; McKiernan, J.M.; Schuckman, A.K. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline: 2024 Amendment. J. Urol. 2024, 211, 533–538. [CrossRef]
[8] Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Dominguez Escrig, J.L.; Gontero, P.; Liedberg, F.; Masson-Lecomte, A.; Mostafid, A.H.; et al. European Association of Urology Guidelines on Non–Muscle-Invasive Bladder Cancer (Ta, T1, and Carcinoma In Situ). Eur. Urol. 2022, 81, 75–94. [CrossRef] [PubMed]
[9] Khansary, S.; Tavilani, H.; Ghasemi, H. Gender, Bladder Cancer Healthcare and Burden of COVID-19. Cancer Investig. 2022, 41, 58–69. [CrossRef]
[10] Tan, W.S.; Steinberg, G.; Witjes, J.A.; Li, R.; Shariat, S.F.; Roupret, M.; Babjuk, M.; Bivalacqua, T.J.; Psutka, S.P.; Williams, S.B.; et al. Intermediate-Risk Non–Muscle-Invasive Bladder Cancer: Updated Consensus Definition and Management Recommendations from the International Bladder Cancer Group. Eur. Urol. Oncol. 2022, 5, 505–516. [CrossRef]
[11] Koch, A.; Joosten, S.C.; Feng, Z.; de Ruijter, T.C.; Draht, M.X.; Melotte, V.; Smits, K.M.; Veeck, J.; Herman, J.G.; Van Neste, L.; et al. Analysis of DNA Methylation in Cancer: Location Revisited. Nat. Rev. Clin. Oncol. 2018, 15, 459–466. [CrossRef] [PubMed]
[12] Kulis, M.; Esteller, M. DNA Methylation and Cancer. Advances in Genetics [Internet] ; Elsevier: New York, NY, USA, 2010; 27–56. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780123808660600022.
[13] Moore, L.D.; Le, T.; Fan, G. DNA Methylation and Its Basic Function. Neuropsychopharmacology 2013, 38, 23–38. [CrossRef] [PubMed]
[14] Law, P.-P.; Holland, M.L. DNA Methylation at the Crossroads of Gene and Environment Interactions. Essays Biochem. 2019, 63, 717–726. [CrossRef]
[15] Martisova, A.; Holcakova, J.; Izadi, N.; Sebuyoya, R.; Hrstka, R.; Bartosik, M. DNA Methylation in Solid Tumors: Functions and Methods of Detection. Int. J. Mol. Sci. 2021, 22. [CrossRef]
[16] Ortega-Recalde, O.; Hore, T.A. DNA Methylation in the Vertebrate Germline: Balancing Memory and Erasure. Essays Biochem. 2019, 63, 649–661. [CrossRef]
[17] Mattei, A.L.; Bailly, N.; Meissner, A. DNA Methylation: A Historical Perspective. Trends Genet. 2022, 38, 676–707. [CrossRef] [PubMed]
[18] Nishiyama, A.; Nakanishi, M. Navigating the DNA Methylation Landscape of Cancer. Trends Genet. 2021, 37, 1012–1027. [CrossRef]
[19] Wu, J.; Lin, Y.; Yang, K.; Liu, X.; Wang, H.; Yu, T.; Tao, R.; Guo, J.; Chen, L.; Cheng, H.; et al. Clinical Effectiveness of a Multitarget Urine DNA Test for Urothelial Carcinoma Detection: A Double-Blinded, Multicenter, Prospective Trial. Mol. Cancer 2024, 23, 1–6. [CrossRef]
[20] Wever, B.; Bach, S.; Tibbesma, M.; ter Braak, T.; Wajon, D.; Dickhoff, C.; Lissenberg-Witte, B.; Hulbert, A.; Kazemier, G.; Bahce, I.; et al. Detection of Non-Metastatic Non-Small-Cell Lung Cancer in Urine by Methylation-Specific PCR Analysis: A Feasibility Study. Lung Cancer 2022, 170, 156–164. [CrossRef]
[21] Guo, R.-Q.; Xiong, G.-Y.; Yang, K.-W.; Zhang, L.; He, S.-M.; Gong, Y.-Q.; He, Q.; Li, X.-Y.; Wang, Z.-C.; Bao, Z.-Q.; et al. Detection of Urothelial Carcinoma, Upper Tract Urothelial Carcinoma, Bladder Carcinoma, and Urothelial Carcinoma with Gross Hematuria Using Selected Urine-DNA Methylation Biomarkers: A Prospective, Single-Center Study. Urol. Oncol. Semin. Orig. Investig. 2018, 36, 342.e15–342.e23. [CrossRef]
[22] Vener, T.; Derecho, C.; Baden, J.; Wang, H.; Rajpurohit, Y.; Skelton, J.; Mehrotra, J.; Varde, S.; Chowdary, D.; Stallings, W.; et al. Development of a Multiplexed Urine Assay for Prostate Cancer Diagnosis. Clin. Chem. 2008, 54, 874–882. [CrossRef]
[23] Fux, R.; Kloor, D.; Hermes, M.; Röck, T.; Proksch, B.; Grenz, A.; Delabar, U.; Bücheler, R.; Igel, S.; Mörike, K.; et al. Effect of Acute Hyperhomocysteinemia on Methylation Potential of Erythrocytes and on DNA Methylation of Lymphocytes in Healthy Male Volunteers. Am. J. Physiol. Physiol. 2005, 289, F786–F792. [CrossRef] [PubMed]
[24] Roobol, M.; Bangma, C.; el Bouazzaoui, S.; Franken-Raab, C.G.; Zwarthoff, E.C. Feasibility Study of Screening for Bladder Cancer with Urinary Molecular Markers (the BLU-P Project). Urol. Oncol. Semin. Orig. Investig. 2010, 28, 686–690. [CrossRef]
[25] Yokoi, K.; Yamashita, K.; Watanabe, M. Analysis of DNA Methylation Status in Bodily Fluids for Early Detection of Cancer. Int. J. Mol. Sci. 2017, 18. [CrossRef] [PubMed]
[26] Trenti, E.; D’Elia, C.; Mian, C.; Schwienbacher, C.; Hanspeter, E.; Pycha, A.; Kafka, M.; Degener, S.; Danuser, H.; Roth, S.; et al. Diagnostic Predictive Value of the Bladder EpiCheck Test in the Follow-Up of Patients with Non-Muscle-Invasive Bladder Cancer. Cancer Cytopathol. 2019, 127, 465–469. [CrossRef] [PubMed]
[27] Oyaert, M.; Van Praet, C.; Delrue, C.; Speeckaert, M.M. Novel Urinary Biomarkers for the Detection of Bladder Cancer. Cancers 2025, 17. [PubMed] [CrossRef]
[28] Magee, D.; Tharakan, N.; Yuiminaga, Y. Validation of Cxbladder® Triage and Monitor as an Adjunct to Urothelial Carcinoma Diagnosis and Surveillance in a Single Centre. Res. Rep. Urol. 2025, ume 17, 87–94. [PubMed] [CrossRef]
[29] Kundal, V.K.; Pandith, A.A.; Hamid, A.; Shah, A.; Kundal, R.; Wani, S.M. Role of NMP22 Bladder Check Test in Early Detection of Bladder Cancer with Recurrence. Asian Pac. J. Cancer Prev. 2010, 11, 1279–1282. [PubMed]
[30] Yao, Z.; Wang, T.; Liu, J.; Zhou, Z.; Zhang, Y. Diagnostic Accuracy of Cytology and Urine Methylation Test in Patients with Non-Muscle Invasive Bladder Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2024, 14. [PubMed] [CrossRef]
[31] Witjes, J.A.; Morote, J.; Cornel, E.B.; Gakis, G.; van Valenberg, F.J.P.; Lozano, F.; Sternberg, I.A.; Willemsen, E.; Hegemann, M.L.; Paitan, Y.; et al. Performance of the Bladder EpiCheck™ Methylation Test for Patients Under Surveillance for Non–Muscle-Invasive Bladder Cancer: Results of a Multicenter, Prospective, Blinded Clinical Trial. Eur. Urol. Oncol. 2018, 1, 307–313. [PubMed] [CrossRef]
[32] Renard, I.; Joniau, S.; van Cleynenbreugel, B.; Collette, C.; Naômé, C.; Vlassenbroeck, I.; Nicolas, H.; de Leval, J.; Straub, J.; Van Criekinge, W.; et al. Identification and Validation of the Methylated TWIST1 and NID2 Genes Through Real-Time Methylation-Specific Polymerase Chain Reaction Assays for the Noninvasive Detection of Primary Bladder Cancer in Urine Samples. Eur. Urol. 2010, 58, 96–104. [CrossRef] [PubMed]
[33] Beijert, I.J.; Wever, B.M.M.; Hentschel, A.E.; Burgt, Y.v.D.; Kauer, P.C.; Lissenberg-Witte, B.I.; van Moorselaar, R.J.A.; Steenbergen, R.D.M.; Nieuwenhuijzen, J.A. Bladder Cancer Detection in Urine by Novel Methylation Markers. Sci. Rep. 2024, 14. [PubMed] [CrossRef]
[34] Thielemans, R.; Speeckaert, R.; Delrue, C.; De Bruyne, S.; Oyaert, M.; Speeckaert, M.M. Unveiling the Hidden Power of Uromodulin: A Promising Potential Biomarker for Kidney Diseases. Diagnostics 2023, 13. [CrossRef] [PubMed]
[35] Jeong, I.G.; Yun, S.-C.; Ha, H.K.; Kang, S.G.; Lee, S.; Park, S.; Sung, H.H.; Kim, S.I.; Hwang, E.C.; Moon, K.C.; et al. Urinary DNA Methylation Test for Bladder Cancer Diagnosis. JAMA Oncol. 2025, 11, 293. [CrossRef] [PubMed]
[36] Bassi, P.; De Marco, V.; De Lisa, A.; Mancini, M.; Pinto, F.; Bertoloni, R.; Longo, F. Non-Invasive Diagnostic Tests for Bladder Cancer: A Review of the Literature. Urol. Int. 2005, 75, 193–200. [PubMed] [CrossRef]
[37] Larsen, L.K.; Lind, G.E.; Guldberg, P.; Dahl, C. DNA-Methylation-Based Detection of Urological Cancer in Urine: Overview of Biomarkers and Considerations on Biomarker Design, Source of DNA, and Detection Technologies. Int. J. Mol. Sci. 2019, 20. [PubMed] [CrossRef]
[38] Bilgrami, S.M.; A Qureshi, S.; Pervez, S.; Abbas, F. Promoter Hypermethylation of Tumor Suppressor Genes Correlates with Tumor Grade and Invasiveness in Patients with Urothelial Bladder Cancer. SpringerPlus 2014, 3, 178. [PubMed] [CrossRef]
[39] Deng, L.; Chao, H.; Deng, H.; Yu, Z.; Zhao, R.; Huang, L.; Gong, Y.; Zhu, Y.; Wang, Q.; Li, F.; et al. A Novel and Sensitive DNA Methylation Marker for the Urine-Based Liquid Biopsies to Detect Bladder Cancer. BMC Cancer 2022, 22. [PubMed] [CrossRef]
[40] Zhang, J.; Chen, J.; Xu, M.; Zhu, T. Exploring Prognostic DNA Methylation Genes in Bladder Cancer: A Comprehensive Analysis. Discov. Oncol. 2024, 15, 331. [CrossRef]
[41] Prip, F.; Lamy, P.; Lindskrog, S.V.; Strandgaard, T.; Nordentoft, I.; Birkenkamp-Demtröder, K.; Birkbak, N.J.; Kristjánsdóttir, N.; Kjær, A.; Andreasen, T.G.; et al. Comprehensive Genomic Characterization of Early-Stage Bladder Cancer. Nat. Genet. 2025, 57, 115–125. [CrossRef]
[42] Xu, Y.; Sun, X.; Liu, G.; Li, H.; Yu, M.; Zhu, Y. Integration of Multi-Omics and Clinical Treatment Data Reveals Bladder Cancer Therapeutic Vulnerability Gene Combinations and Prognostic Risks. Front. Immunol. 2024, 14. [CrossRef] [PubMed]
[43] Cao, L.; Ma, X.; Rong, P.; Zhang, J.; Yang, M.; Wang, W. Comprehensive Analysis of DNA Methylation and Transcriptome to Identify PD-1-Negative Prognostic Methylated Signature in Endometrial Carcinoma. Dis. Markers 2022, 2022, 1–24. [CrossRef] [PubMed]
[44] Lee, K.H. Evaluation of the NMP22 Test and Comparison with Voided Urine Cytology in the Detection of Bladder Cancer. Yonsei Med. J. 2001, 42, 14–18. [CrossRef] [PubMed]
[45] Hentschel, A.E.; Beijert, I.J.; Bosschieter, J.; Kauer, P.C.; Vis, A.N.; Lissenberg-Witte, B.I.; van Moorselaar, R.J.A.; Steenbergen, R.D.M.; Nieuwenhuijzen, J.A. Bladder Cancer Detection in Urine Using DNA Methylation Markers: A Technical and Prospective Preclinical Validation. Clin. Epigenetics 2022, 14, 19. [CrossRef]
[46] Leng, D.; Zheng, L.; Wen, Y.; Zhang, Y.; Wu, L.; Wang, J.; Wang, M.; Zhang, Z.; He, S.; Bo, X. A Benchmark Study of Deep Learning-Based Multi-Omics Data Fusion Methods for Cancer. Genome Biol. 2022, 23. [CrossRef]
[47] Alharbi, F.; Vakanski, A.; Elbashir, M.K.; Mohammed, M. LASSO–MOGAT: A Multi-Omics Graph Attention Framework for Cancer Classification. Acad Biol. 2024, 2. [CrossRef]
We use cookies to improve your experience on our site. By continuing to use our site, you accept our use of cookies. Learn more