
APA Style
Ao Gu, Jiatong Li, Nannan Tang, Gökhan Zengin, Meng-Yao Li. (2025). Food and Medicine Homology Substance in Cancer Treatment: Mechanism of Astragalus against Pancreatic Cancer. Molecular Modeling Connect, 2 (Article ID: 0004). https://doi.org/10.69709/MolModC.2025.131915MLA Style
Ao Gu, Jiatong Li, Nannan Tang, Gökhan Zengin, Meng-Yao Li. "Food and Medicine Homology Substance in Cancer Treatment: Mechanism of Astragalus against Pancreatic Cancer". Molecular Modeling Connect, vol. 2, 2025, Article ID: 0004, https://doi.org/10.69709/MolModC.2025.131915.Chicago Style
Ao Gu, Jiatong Li, Nannan Tang, Gökhan Zengin, Meng-Yao Li. 2025. "Food and Medicine Homology Substance in Cancer Treatment: Mechanism of Astragalus against Pancreatic Cancer." Molecular Modeling Connect 2 (2025): 0004. https://doi.org/10.69709/MolModC.2025.131915.
 ACCESS
Research Article
ACCESS
Research Article
Volume 2, Article ID: 2025.0004

Ao Gu
guao4826@sjtu.edu.cn

Jiatong Li
lijiatong@renji.com

Nannan Tang
tangnannan@renji.com

Gökhan Zengin
gokhanzengin@selcuk.edu.tr

Meng-Yao Li
limy@sioc.ac.cn
1 State Key Laboratory of Systems Medicine for Cancer, Shanghai Cancer Institute, Renji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200127, China
2 Department of Biliary-Pancreatic Surgery, Renji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200127, China
3 Shanghai Key Laboratory for Cancer Systems Regulation and Clinical Translation, Shanghai Jiading District Central Hospital, Shanghai 201800, China
4 Department of Biology, Science Faculty, Selcuk University, Konya 42130, Turkey
* Author to whom correspondence should be addressed
Received: 23 Nov 2024 Accepted: 09 Mar 2025 Available Online: 10 Mar 2025 Published: 20 Mar 2025
This work explores the mechanism of food and medicine homology substance—astragalus—against pancreatic cancer (PC). PC is a notably lethal solid tumor, often presenting with a poor clinical prognosis and typically diagnosed at an advanced stage. Food and medicine homology herbs possess dual attributes as both functional foods and therapeutic agents, which showed promise as a treatment strategy demonstrating substantial inhibitory effects with reduced side effects and toxicity about PC. Astragalus (HQ, also known as Huang-Qi in Chinese), a herb that falls under the category of food and medicine homology, shows potential in combating PC. To explore its underlying mechanism, we utilized network pharmacology to identify potential compounds and their related targets of PC and molecular docking techniques to assess the binding affinity between the identified compound ds and targets. The presence of quercetin, kaempferol, isorhamnetin, and formononetin in HQ provides compelling evidence for their pivotal role as key constituents in combating PC. Our findings suggest that HQ potentially regulated MMP-9 through TNF/TGF, thereby exerting its inhibitory effects on PC. This study elucidates a novel mechanism by which a natural herb exhibits anti-PC capacity.
Pancreatic cancer (PC) is a condition associated with a poor clinical prognosis, resulting in high mortality rates among patients [1,2,3]. The primary treatment methods currently utilized encompass surgery, chemotherapy [4], and radiotherapy [5]. Although targeted therapy [6,7] and immunotherapy [8,9,10] hold potential promise for PC patients, their benefits are still limited [11]. Therefore, there is an urgent need to develop alternative therapeutic strategies that can reduce adverse effects and enhance overall patient survival [12,13,14,15,16,17,18]. Traditional Chinese Medicine (TCM) has emerged as a valuable supplementary therapy, offering promising potential for the development of drugs through its multi-compound, multi-target, and multi-pathway approach [19,20,21]. TCM has shown significant anti-cancer effects and has been extensively researched for its potential in cancer treatment. Herbal medicines are typically cost-effective, widely accessible, and exhibit minimal toxicity or adverse effects in clinical settings [22,23]. However, despite the substantial interest and growing demand, the lack of robust evidence-based research and the absence of standardized herbal product formulations pose significant challenges to the global dissemination of TCM. In recent years, advancements in analytical technologies and methodologies have significantly propelled research in TCM [24,25,26,27]. Food and medicine homology (FMH) has been a cornerstone in TCM since ancient times, underscoring the inherent link and mutual transformation between food and drugs [28]. FMH substances are characterized by their dual functionality; they serve as nutritional sources and medicinal agents [29]. These substances not only alleviate hunger but also demonstrate pharmacological effects when preventing and treating disease [30,31]. There is a growing trend where individuals are turning to dietary practices for health regulation, often preferring “food therapy” over “medication therapy”. [32,33,34] Individuals tend to prefer functional foods [35] or beverages [36] as a method of bolstering their immune system against diseases, rather than depending on nutritional supplements [37]. Long-term ingestion of these substances is associated with beneficial health outcomes, disease prevention, and minimal adverse effects due to their nutritional and therapeutic properties, as well as their negligible toxicity [38,39]. Astragalus (also known as Huang-Qi in Chinese and Hedysarum Multijugum Maxim in Latin, HQ) is a potential anti-tumor herb. It is also renowned for its anti-aging and immunomodulating properties [40,41,42]. Previous studies have reported the antitumor effect of HQ on hepatocellular carcinoma (HCC). The combination with Atractylodes (also known as Bai-Zhu in Chinese and Atractylodes Macrocephala Koidz in Latin, BZ) has demonstrated potential in the treatment of HCC, specifically through targeting IL-6/STAT3 and modulating immune cell activity [43]. Additionally, HQ has demonstrated potential in treating breast cancer [44], colorectal cancer [45], and prostate cancer. However, further research is needed to explore the potential antitumor effects of HQ as a standalone component, as existing studies have primarily focused on medicinal formulations containing two or more ingredients. The single specific mechanism of HQ has been shown to exhibit anti-cancer effects on cell proliferation [46], apoptosis [47], and metastasis [48]. In this regard, HQ may offer potential therapeutic options for patients with PC, and further investigation is warranted to explore its efficacy and related mechanisms. Network pharmacology could be considered an invaluable tool, leveraging bioinformatics data to explore biological systems and pinpoint potential targets of TCMs [49]. As the comprehension of cancer’s intricate nature expands, the constraints of singularly targeted therapies are increasingly evident. Network pharmacology, emerging as a pioneering research methodology, provides insights into the underlying mechanisms of TCM in cancer therapy by adopting a multi-target and multi-pathway lens. Furthermore, this method illuminates the synergistic impacts of TCM compounds through the construction of drug-target-disease networks. This not only offers a novel perspective on the application of TCM in cancer management but also catalyzes the modernization of TCM and propels the evolution of precision medicine. In this study, network pharmacology was employed to utilize public databases for identifying shared targets between HQ and PC, validating their potential targets and compounds. Subsequently, molecular docking analysis was conducted to confirm their affinity further. This study provides a fundamental basis for investigating the potential use of FMH herbs in cancer treatment.
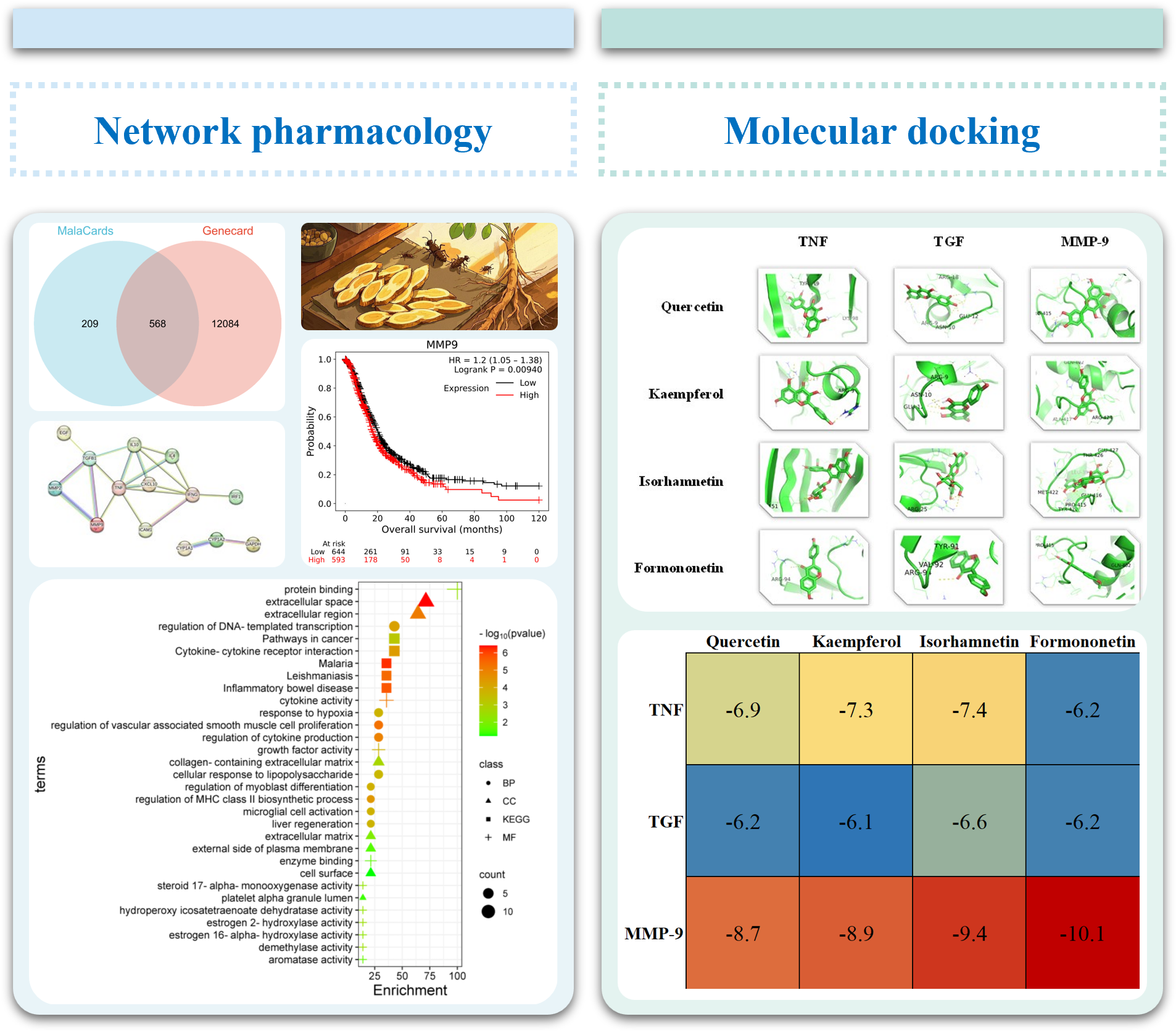
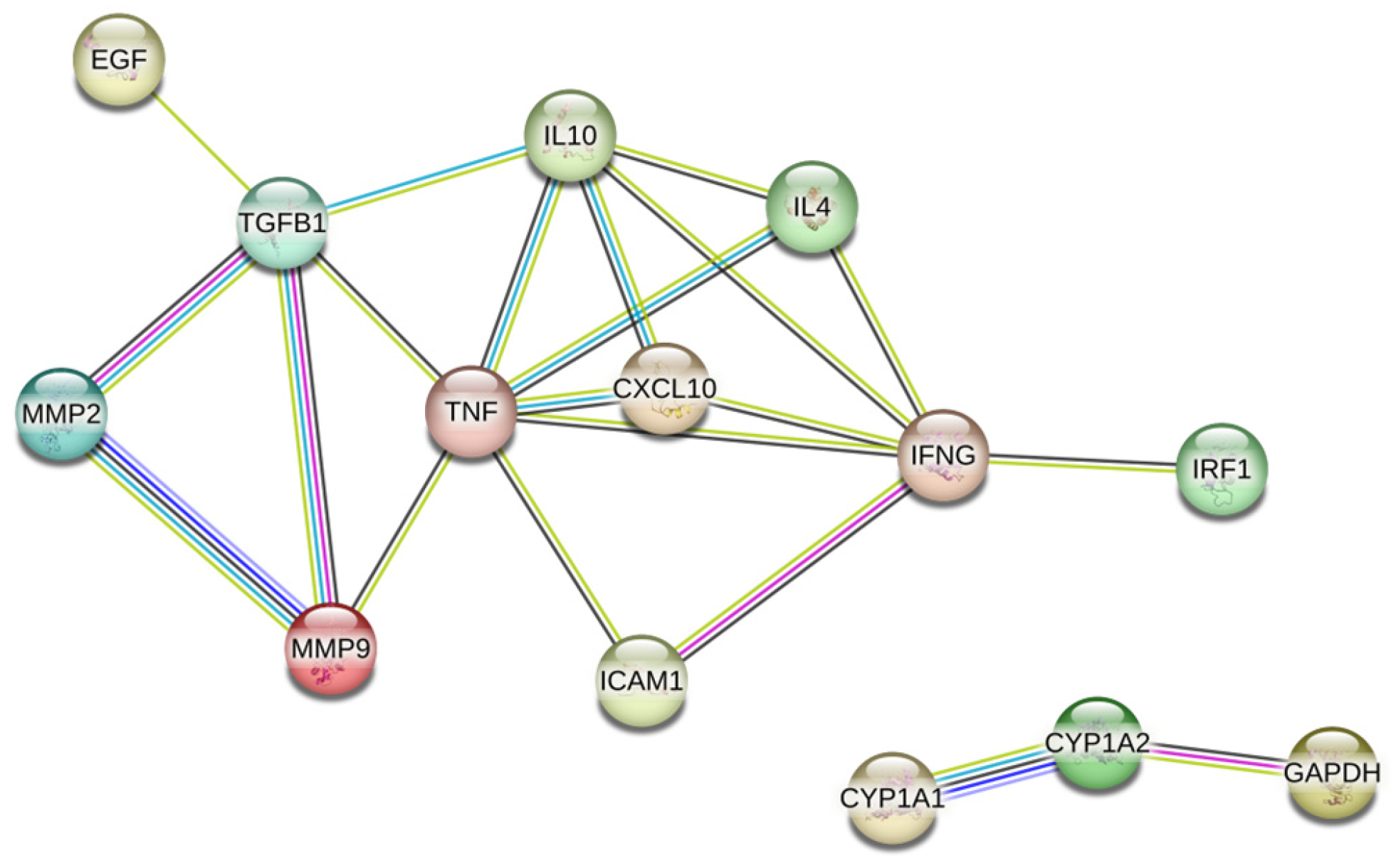
2.1. Collecting HQ Potential Targets HQ putative targets were obtained from TCMSP databases “https://old.tcmsp-e.com/tcmsp.php (accessed on 20 November 2024)”. The UniProt database “https://www.uniprot.org/ (accessed on 20 November 2024)” was used to translate the protein names into corresponding gene symbols. 2.2. Identification of PC-Related Therapeutic Genes In order to screen for potential therapeutic targets for PC, the keyword “pancreatic cancer” was utilized in two public databases: MalaCards “https://www.malacards.org (accessed on 12 November 2024)” and GeneCards “https://www.genecards.org/ (accessed on 12 November 2024)”. Subsequently, common targets within these databases were identified using Venn diagrams. 2.3. Potential Targets Identification The overlapping targets were identified using Venn diagrams, which compared the HQ potential targets with PC-related therapeutic genes. 2.4. protein-Protein Interaction (PPI) Analysis First, the targets were input into the STRING database “https://string-db.org/cgi/input.pl (accessed on 12 November 2024)”, and the species was set as Homo sapiens. Then, the interaction analysis results were exported, and the protein interaction degree was used for subsequent analysis. 2.5. Enrichment Analyses Gene Ontology (GO) enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis were conducted. The visualization of signaling pathway diagrams was facilitated by the KEGG “https://www.kegg.jp/ (accessed on 12 November 2024)”. 2.6. Network Construction The network of herb-compound targets was meticulously constructed utilizing the advanced capabilities of Cytoscape. After this construction, a comprehensive degree analysis was conducted to facilitate an in-depth examination and elucidation of the intricate relationships within the network. 2.7. Molecular Docking Analysis Four hub genes, which may play crucial roles in mediating the effects of HQ on PC, were identified based on their degree of centrality in the PPI network and selected for molecular docking simulations with HQ constituents. The HQ constituents were selected through the herb-compound-targets network based on their highest degree values. These simulations were carried out using AutoDock Tools version 1.5.6, as previously described [50]. The protein structures were sourced from the Protein Data Bank [PDB, “http://www.rcsb.org/ (accessed on 24/11/2024)”] and were pre-processed using PyMOL software to eliminate water molecules, co-crystallized ligands, and ions. The docking results were saved as protein files in PDBQT format. [51]. 2.8. Prognostic Analyses of Potential Therapeutic Target Genes Survival analyses of potential therapeutic target genes MMP-9 were conducted on the Cancer Genome Atlas (TCGA) database. Kaplan-Meier survival analyses, utilizing the “surv_cutpoint” function from the survminer package, were conducted to determine the risk scores’ cut-off value.
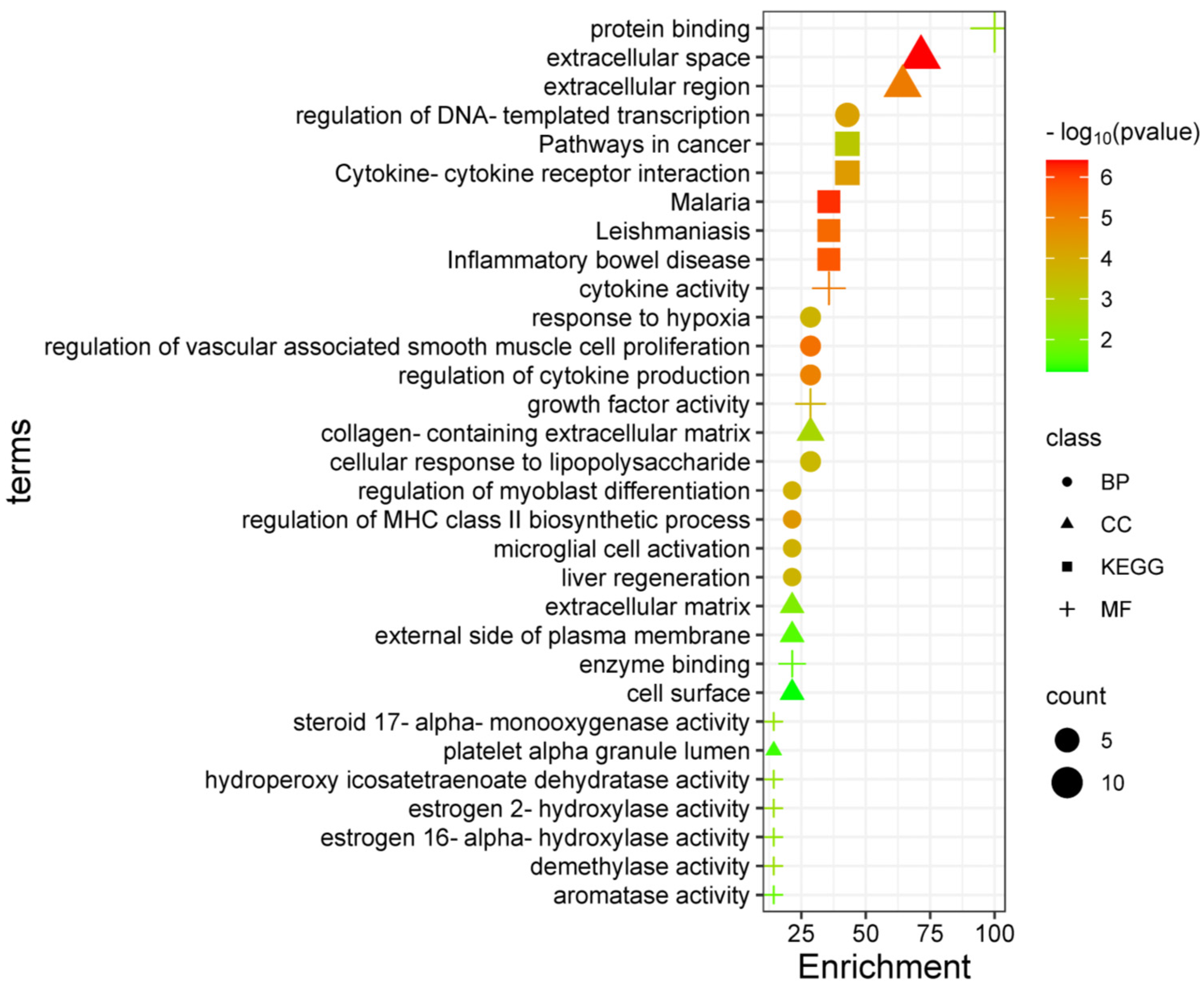
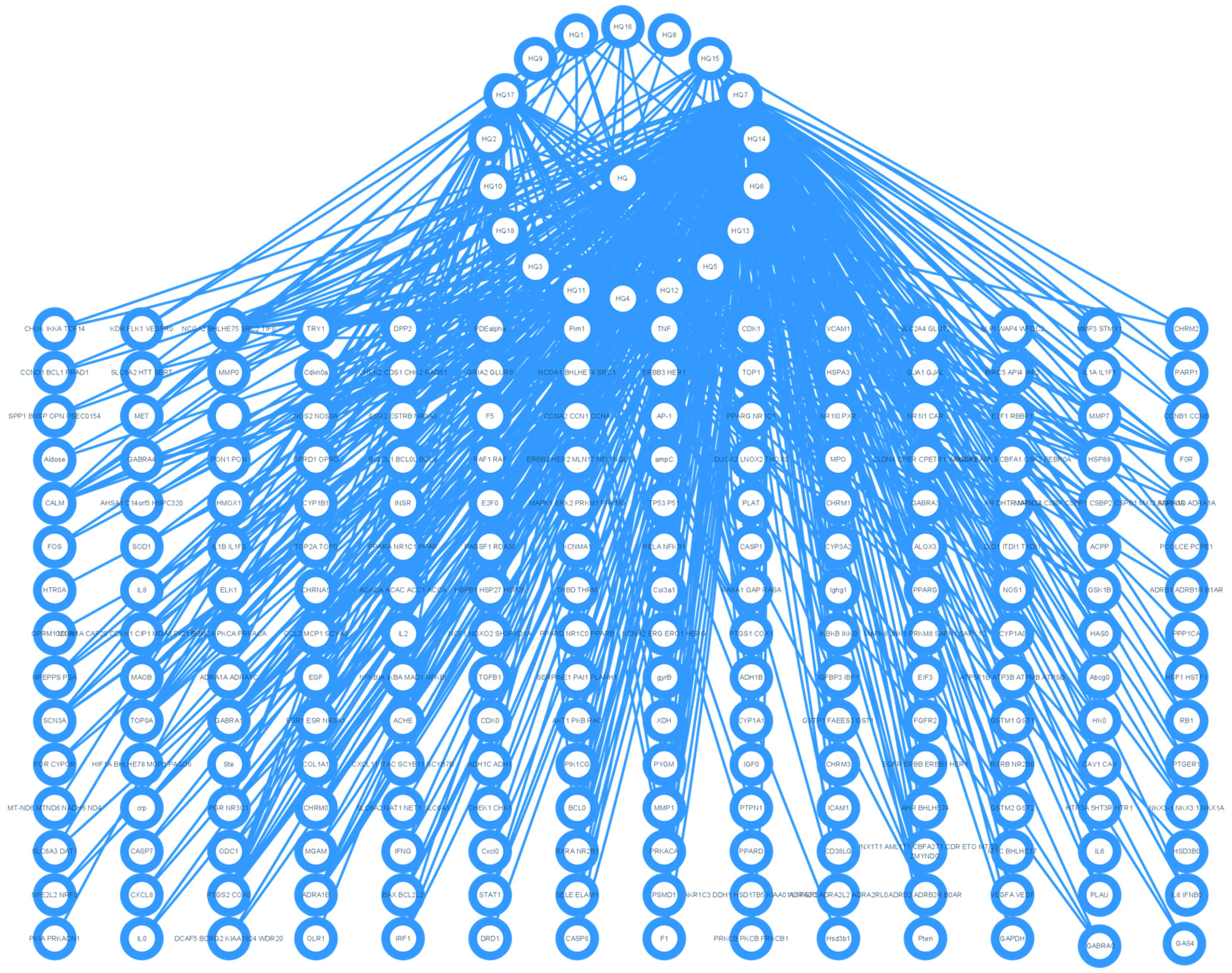
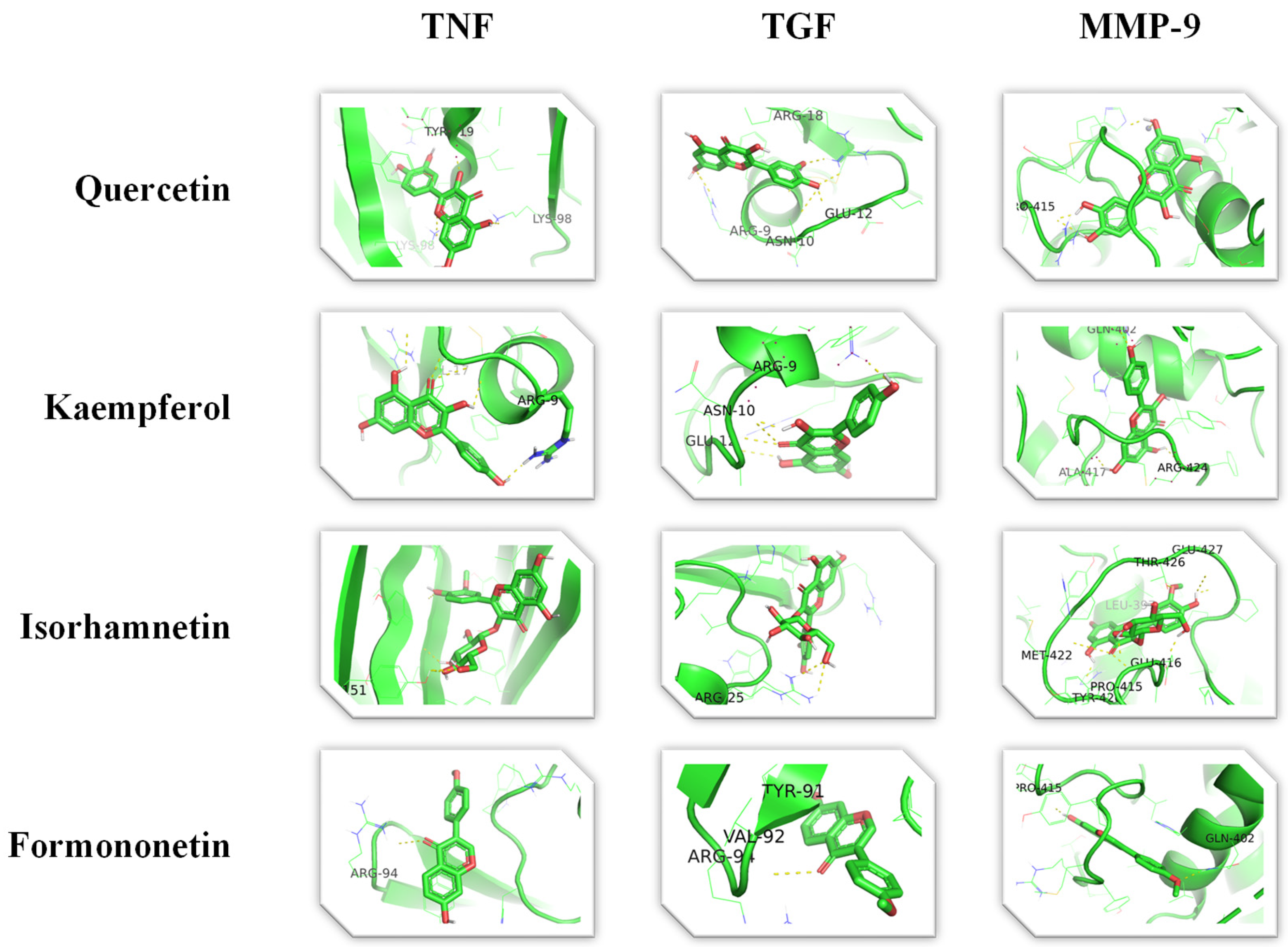
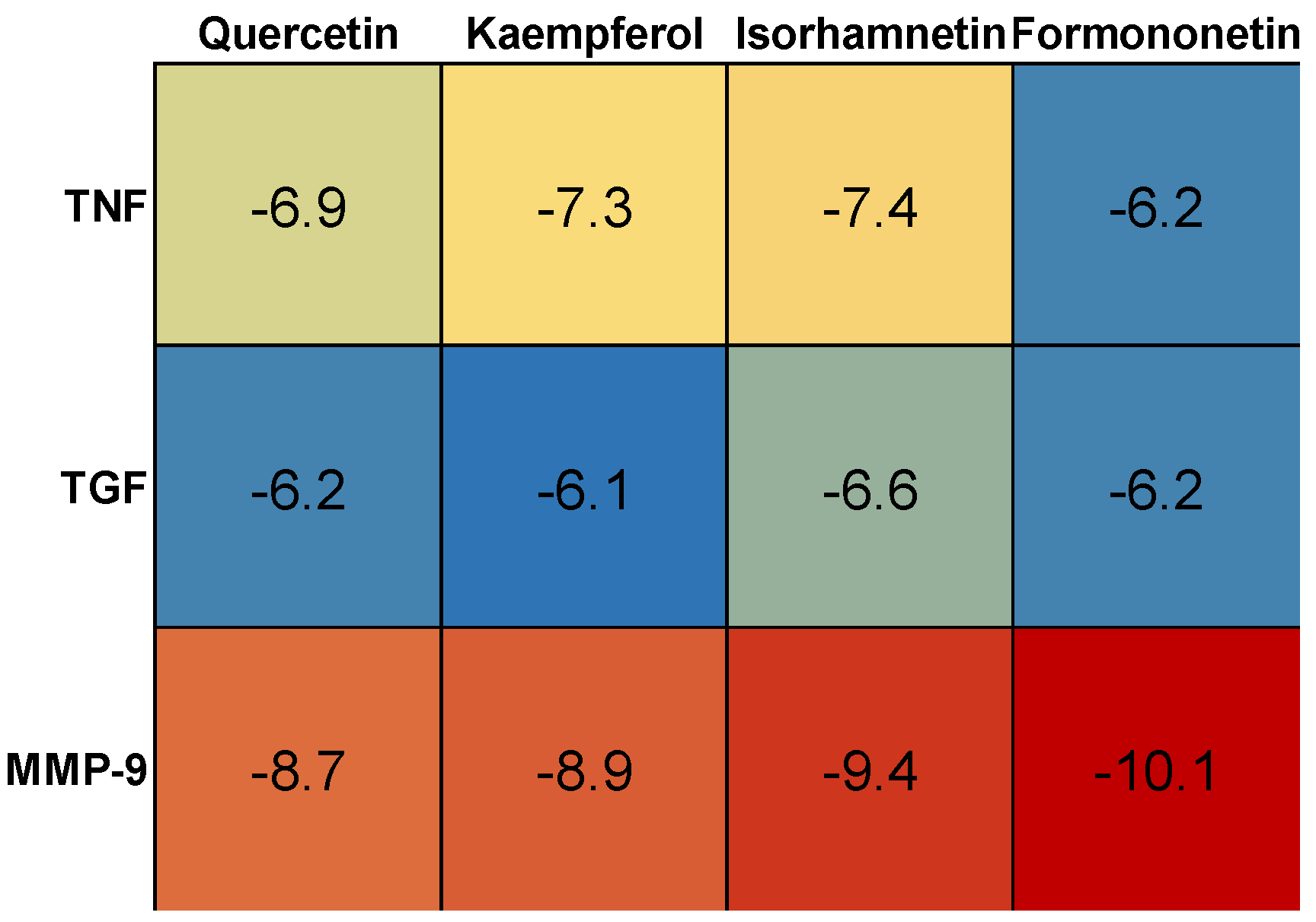
3.1. Target Genes of HQ The potential target genes associated with HQ were sourced from the TCMSP databases, yielding 16 compounds and 211 therapeutic targets. 3.2. PC-Related Target Genes Initially, we performed an exhaustive search in two databases using the keyword “Pancreatic cancer,” which yielded 777 targets in MalaCards and 12,652 targets in GeneCards. Moreover, we identified overlapping targets and converted protein names into gene symbols, resulting in a total of 568 putative targets associated with PC (Figure 1). 3.3. Construction and Topological Analysis of the PPI Network The 24 genes linked with HQ-PC were scrutinized utilizing the STRING database (Figure 2), which led to the creation of a PPI network with a minimum interaction score threshold set at 0.9. Following this, ten potential gene targets were singled out for additional examination, based on their protein degree. TNF, TGF, and MMP-9 were confirmed as the central target genes for further analysis (Figure 3). 3.4. GO Enrichment and KEGG Analysis GO enrichment analysis was performed to explore the potential targets, including biological processes (BP), cellular components (CC), and molecular functions (MF). A total of 158 GO terms were obtained, among which 134 BP, 7 CC, and 17 MF. The bubble diagram (Figure 4) showed the top ten significantly enriched entries for BP, CC, and MF with the smallest p-value. The redder color of the dot indicated the smaller p-value and more significant enrichment of the corresponding term. The bigger dot represented the higher gene count in each entry. The analysis results revealed that the commonly targeted genes of HQ and PC are primarily enriched in several biological processes. These include positive regulation of mononuclear cell migration (GO: 0071677), vascular-associated smooth muscle cell proliferation (GO: 1904707), cytokine production (GO: 0001819), MHC class II biosynthetic process (GO: 0045348), DNA-templated transcription (GO: 0045893), myoblast differentiation (GO: 0045662), microglial cell activation (GO: 0001774), liver regeneration (GO: 0097421), response to hypoxia (GO: 0001666), and cellular response to lipopolysaccharide (GO: 0071222). Additionally, these genes are associated with various cell components such as the CC extracellular space (GO: 0005615), extracellular region (GO: 0005576), collagen-containing extracellular matrix (GO: 0062023), extracellular matrix (GO: 0031012), external side of the plasma membrane (GO: 0009897), platelet alpha granule lumen (GO: 0031093), and cell surface (GO: 0009986). Furthermore, they are involved in molecular functions like cytokine activity (GO: 0005125), growth factor activity (GO: 0008083), demethylase activity (GO: 0032451), protein binding (GO: 0005515), hydroperoxy icosatetraenoate dehydratase activity (GO: 0106256), steroid 17-alpha-monooxygenase activity (GO: 0004508), estrogen 16-alpha-hydroxylase activity (GO: 0101020), aromatase activity (GO: 0070330), and enzyme binding (GO: 0019899). 3.5. KEGG Enrichment Analysis of Co-Targeted Genes of HQ and PC The KEGG analysis was utilized to explore the potential functions and signaling pathways of the identified anti-PC targets associated with HQ. The results from the KEGG pathway analysis indicated that 10 target genes were notably enriched, leading to a total of 54 statistically significant pathways. The top five pathways with the highest gene counts selected are: Cytokine-cytokine receptor interaction (hsa04060, n = 6), Pathways in cancer (hsa05200, n = 6), IL-17 signaling pathway (hsa0465, n = 5), TNF signaling pathway (hsa04668, n = 5), and Fluid shear stress and atherosclerosis (hsa05418, n = 5). 3.6. Construction of Compound-Targets Network To elucidate the interplay among targets, compounds, and HQ, a network encompassing HQ-compounds-targets was meticulously constructed utilizing Cytoscape. Through an in-depth degree analysis, it was unequivocally confirmed that quercetin assumes the role of the principal compound within HQ during the anti-PC process. Furthermore, kaempferol, isorhamnetin, and formononetin were identified as the most promising compounds within HQ (Figure 5). 3.7. Molecular Docking Based on the PPI and the network degree analysis, the molecular docking analysis of the compound’s quercetin, kaempferol, isorhamnetin, and formononetin with TNF, TGF, and MMP-9 was conducted to ascertain their affinity (Figure 6). The most significant combination was observed between formononetin and MMP-9, exhibiting an affinity of -10.11 kcal/mol. Conversely, the least binding energy was noted between kaempferol and TGF, registering an affinity of -6.1 kcal/mol (Table 1). The binding affinity of MMP-9 to the four small molecules was significantly higher compared to their affinity towards TNF and TGF, suggesting a potential direct interaction between these molecules and MMP-9 protein, subsequently leading to indirect regulation of TNF and TGF (Figure 7). These results were validated through the previous study [52], formononetin showed decreased regulation of MMP-9 in breast cancer, which was confirmed associated with metastatic PC [53]. Quercetin was shown to improve the disease prognosis by inhibiting MMP-9 [54]. Isorhamnetin was reported to potentially inhibit the proliferation and metastasis of cancer cells [55]. Kaempferol was investigated and found to inhibit cancer cells’ migration and invasion [56,57]. Molecule docking data results. 3.8. Prognostic Analyses of Potential Therapeutic Target Genes Based on the TCGA database, which contains various available comprehensive cancer types data, the prognosis of PC patients expressing MMP-9 was investigated. This analysis revealed that MMP-9 downregulation was correlated with improved survival in PC patients. (Figure 8).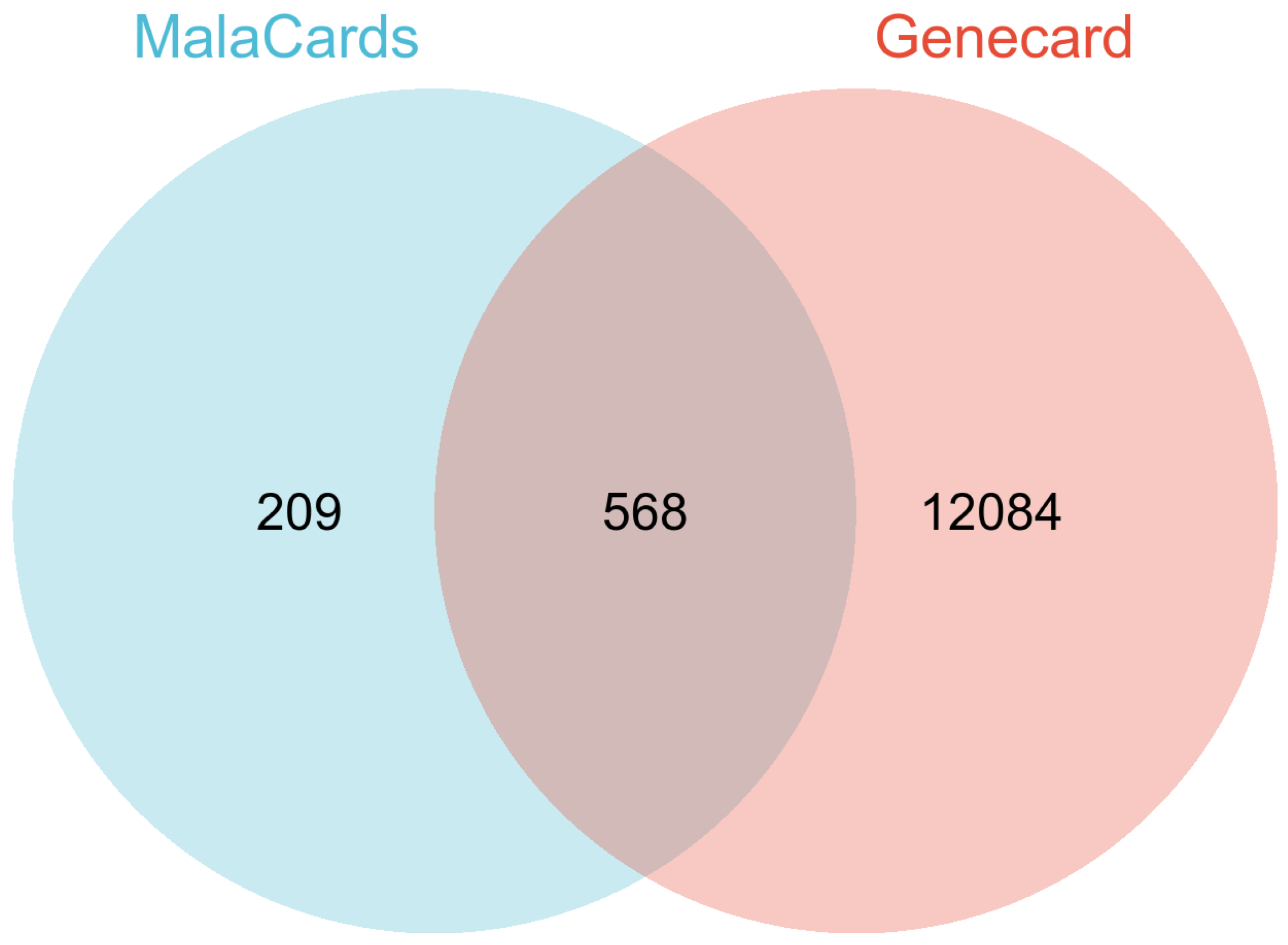
Targets
Compounds
Binding Site
Affinity (kcal/mol)
Estimated Ki
Ligand Efficiency
TNF
Quercetin
2
−6.9
8.76 μM
−0.31
Kaempferol
9
−7.3
4.46 μM
−0.35
Isorhamnetin
9
−7.4
3.77 μM
−0.22
Formononetin
7
−6.2
28.53 μM
−0.31
TGF
Quercetin
2
−6.2
28.53 μM
−0.28
Kaempferol
5
−6.1
33.78 μM
−0.29
Isorhamnetin
6
−6.6
14.53 μM
−0.19
Formononetin
4
−6.2
28.53 μM
−0.31
MMP-9
Quercetin
8
−8.67
0.44 μM
−0.39
Kaempferol
8
−8.88
0.31 μM
−0.42
Isorhamnetin
2
−9.36
0.14 μM
−0.28
Formononetin
1
−10.11
38.84 nM
−0.51
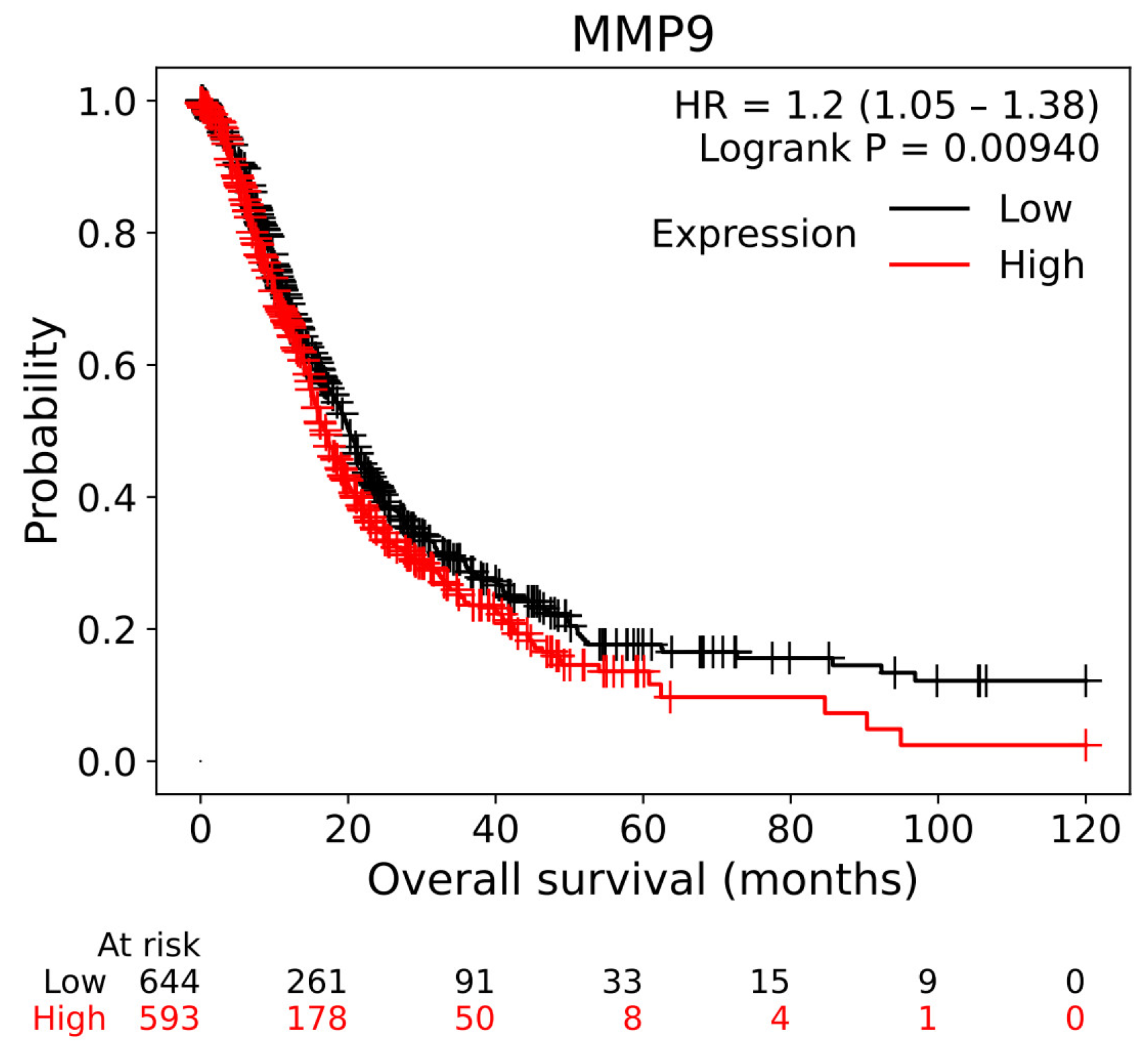
PC is a lethal disease that presents significant challenges in the development of effective therapeutic strategies [58,59]. This study employed a combination of network pharmacology and molecular docking to explore the potential therapeutic benefits of HQ in treating PC. The bioactive components of HQ and their potential PC-targeted genes were thoroughly analyzed, underscoring the multifaceted therapeutic effects of various compounds. Utilizing the TCMSP database, we identified 16 active compounds and 211 therapeutic targets that demonstrated favorable oral bioavailability (OB) and drug-likeness (DL). Upon further analysis of these 211 genes to discern common targets, we found 24 overlapping targets within the MalaCards and GeneCards database, encompassing a total of 568 PC genes. This suggests that the therapeutic efficacy of HQ may be attributed to its multi-component nature and its involvement in multiple molecular pathways, underscoring a key characteristic of TCMs. The PPI network analysis, constructed using a stringent confidence threshold, was employed to examine the top ten-degree core target genes. Subsequently, these selected core targets underwent GO and KEGG analysis, yielding 158 GO terms and 54 KEGG terms. The observed complexity suggests that the active compounds in HQ concurrently influence multiple biological processes, offering a potential strategy for addressing the heterogeneity of PC. GO enrichment highlighted the biological processes, cell components, and molecular functions of these gene targets when engaged and absorbed by cancer cells. These include regulation of mononuclear cell migration, response to hypoxia, and protein binding, which align with the current understanding of PC progression. This conforms to its anti-PC mechanism and enhances understanding at the molecular level [60]. The enriched KEGG terms from the selected proteins suggest that the potential compounds of HQ are likely to exert an anti-tumor effect through multiple signaling pathways. These are particularly associated with Cytokine-cytokine receptor interaction, Pathways in cancer, IL-17 signaling pathway, and TNF signaling pathway. Chronic systemic and localized inflammation may heighten the risk of PC. The inflammatory infiltrate linked with PC within the tumor microenvironment contributes to the promotion of tumor growth and metastasis. Inflammation is intricately intertwined with the immune system, with the same immune cell populations playing roles in both inflammatory processes and immune responses [61]. The results of the KEGG analysis reveal that HQ may exert its therapeutic effects by targeting the inflammatory process associated with PC progression. This suggests an alternative treatment strategy focused on modulating inflammation-related target genes [62]. The herb-compound-targets network analysis was utilized to pinpoint the most efficacious compound for anti-PC applications. The compounds quercetin, kaempferol, isorhamnetin, and formononetin were identified as possessing the highest potential. These compounds are all flavonoids, exhibiting significant anti-tumor and antioxidant properties [63,64,65,66]. They have the ability to neutralize active oxygen by inhibiting free radical generation and can potentially mitigate inflammation [67]. A plethora of studies have shown that quercetin can impede the growth and migration of pancreatic cancer cells through various mechanisms. Upon treatment with quercetin, the cell cycle of pancreatic cancer cells is arrested in the S phase, achieved by downregulating cyclin A expression [68]. Furthermore, quercetin can reduce the invasiveness of PC cells by suppressing the expression of MMP genes [69]. Additionally, it can trigger mitochondrial-dependent apoptosis in pancreatic cancer cells by inhibiting the PI3K/Akt signaling pathway [70]. Studies revealed that kaempferol can suppress the Akt/mTOR signaling pathway, thereby augmenting its anti-PC properties [71]. Furthermore, it has been demonstrated to induce cell cycle arrest, specifically in the G0/G1 phase, thus inhibiting the proliferation of cancer cells [72]. Isorhamnetin, an O-methylated derivative of quercetin, exhibits properties analogous to quercetin in the context of pancreatic cancer treatment [73]. Formononetin has been shown to increase the efficacy of chemotherapeutic agents, which can enhance the anti-proliferative activity of gemcitabine, a standard treatment for pancreatic cancer, by downregulating key survival pathways [74,75]. This synergistic effect suggests that formononetin may be useful as an adjunct therapy in the treatment of pancreatic cancer. The molecular docking analysis indicated that the most promising binding pair was formononetin and MMP-9, exhibiting a binding affinity of -10.12 kcal/mol. Kaempferol and MMP-9 demonstrated a binding affinity of -8.88 kcal/mol, while quercetin and MMP-9 showed a slightly lower affinity of -8.67 kcal/mol. Notably, isorhamnetin displayed the highest binding affinity among all tested compounds, with an impressive value of -9.36 kcal/mol against MMP-9. These results collectively underscore the pivotal role of MMP-9 in mediating the anti-PC effects of HQ. MMP-9 has been correlated with PC cell invasion [76], migration [77], and growth [78]. The increased expression of MMPs in pancreatic cancer, combined with the universally poor survival rates associated with even relatively early-stage disease, complicates the establishment of clinicopathologic correlations [79]. TNF /TGF was found to be associated with poor prognosis of PC [80], and inhibiting the TNF/TGF can inhibit the epithelial-to-mesenchymal transition of PC [81]. Therefore, by downregulating the TNF/TGF and MMP-9, we may find patients with a better prognosis. There is substantial evidence demonstrating the regulatory role of TGF and TNF interaction in modulating MMP-9, thereby influencing breast cancer progression [82]. This suggests that HQ may modulate the progression of pancreatic cancer through the TGF/TNF pathway to regulate MMP-9 signaling. However, some limitations still exist in this study. The investigation of the potential compounds still lies in the in-silico aspect, further validation based on the in vitro and in vivo models is needed. The exploration of anti-tumor compounds is built upon previous studies, yet there may be undiscovered effective compounds that remain to be identified, necessitating further research. Future research should focus on enhancing clinical relevance, which requires validation through patient-derived models that best preserve the structural and mutational characteristics of patients’ tumors [83].
In conclusion, this study utilizes a network pharmacology analysis and molecular docking to reveal the potential therapeutic mechanisms of HQ in treating PC. The identification of active compounds and their associated targets provides a robust foundation for subsequent in vitro and in vivo experiments. A wealth of evidence suggests that quercetin, kaempferol, isorhamnetin, and formononetin are effective anti-pancreatic cancer ingredients present in HQ. These compounds have the potential to regulate MMP-9 through the TNF/TGF axis. This pioneering study illuminates the unprecedented potential effect and mechanism of HQ against pancreatic cancer, offering a fresh perspective on the exploration of food and medicine homology herbs for tumor therapy. Future research should focus on validating these potential mechanisms using preclinical models while investigating their clinical translational potential. However, further validation is necessary, and patient-derived models should serve as a robust platform to evaluate their clinical potentials.
| PC | Pancreatic cancer |
| TCM | Traditional Chinese medicine |
| MFH | medicine and food homology |
| GO | Gene ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| PPI | protein-protein interaction |
| BP | biological processes |
| CC | cellular components |
| MF | molecular functions |
M.-Y.L. and G.Z. conceived the work; A.G. designed and conducted the experiments; A.G. wrote the manuscript. A.G., J.L., N.T., G.Z., and M.-Y.L. revised the manuscript. All authors analyzed the results and commented on the manuscript.
Data supporting the results of this study are available upon request from the corresponding author.
The study has no ethical implications.
The research didn’t involve any human subjects.
Not Applicable.
The authors declare no conflicts of interest.
No external funding was received for this research.
We acknowledge Shiyanjia Lab (www.shiyanjia.com) for their language editing services.
[1] Advancing on Pancreatic Cancer. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 447. [CrossRef] [PubMed]
[2] Gurav, S.; Singh, G.P.; Ostwal, V.; Sharma, M.; Jagtiani, K. Metastatic pAncreatic Cancer Mimicking Medication Related Osteonecrosis of the Jaw—A Rare Clinical Presentation. Med. Adv. 2023, 1, 408–412. [CrossRef]
[3] Mustafa, J.; Sebastine, A.G.; Sazgar, I.; Nadeem, T. Recent Advances in Detecting Premalignant Pancreatic Cysts. J. Cancer Metastasis Treat. 2023, 9, 11. [CrossRef]
[4] Li, J.; Gu, A.; Nong, X.M.; Zhai, S.; Yue, Z.Y.; Li, M.Y.; Liu, Y. Six-Membered Aromatic Nitrogen Heterocyclic Anti-Tumor Agents: Synthesis and Applications. Chem. Rec. 2023, 23, e202300293. [CrossRef]
[5] Wood, L.D.; Canto, M.I.; Jaffee, E.M.; Simeone, D.M. Pancreatic Cancer: Pathogenesis, Screening, Diagnosis, and Treatment. Gastroenterology 2022, 163, 386–402.e381. [CrossRef]
[6] Shubhra, Q.T.H.; Cai, X.; Cai, Q. Next-Generation Tumor Targeting with Genetically Engineered Cell Membrane-Coated Nanoparticles. BioDesign Res. 2024, 6. [CrossRef] [PubMed]
[7] Huang, X.; Cao, X. Innovative Drugs Bring Continuous Benefits to Cancer Patients. Innov. Life 2023, 1, 100043. [CrossRef]
[8] Qian, K.; Li, G.; Zhang, S.; Fu, W.; Li, T.; Zhao, J.; Lei, C.; Wang, Y. CAR-T-Cell Products in Solid Tumors: Progress, Challenges, And Strategies. Interdiscip. Med. 2024, 2, e20230047. [CrossRef]
[9] Xu, G.; Li, J.; Zhang, S.; Cai, J.; Deng, X.; Wang, Y.; Pei, P. Two-Dimensional Nano-Biomaterials in Regulating the Tumor Microenvironment for Immunotherapy. Nano TransMed. 2024, 3, 100045. [CrossRef]
[10] Zhang, H.; Chen, J.; Zhang, Q.; Yu, L.; Li, X.; Wu, S. Oncogenic Viral Antigens for Engineered T Cell Immunotherapy: Challenges and Opportunities. Med. Adv. 2023, 1, 306–317. [CrossRef]
[11] Gu, A.; Li, J.; Li, M.-Y.; Liu, Y. Patient-Derived Xenograft Model in Cancer: Establishment and Applications. MedComm 2025, 6, e70059. [CrossRef] [PubMed]
[12] Li, J.-T.; Gu, A.; Tang, N.-N.; Sun, Z.-Y.; Zhang, G.; Li, M.-Y. Exploring Anti-Tumor Potential of Food and Medicine Homology Substances: An In-Silico Evaluation of Citri Grandis Exocarpium Against Gallbladder Cancer. Food Med. Homol. 2026, 3, 9420084. [CrossRef]
[13] Wang, J.; Liao, Z.-X. Research Progress of Microrobots in Tumor Drug Delivery. Food Med. Homol. 2024, 1, 9420025. [CrossRef]
[14] Wang, X.; Mao, K.; Zhang, X.; Zhang, Y.; Yang, Y.-G.; Sun, T. Red Blood Cell Derived Nanocarrier Drug Delivery System: A Promising Strategy for Tumor Therapy. Interdiscip. Med. 2024, 2, e20240014. [CrossRef]
[15] Liguori, F.; Pellicciotta, N.; Milanetti, E.; Xi Windemuth, S.; Ruocco, G.; Di Leonardo, R.; Danino, T. Dynamic Gene Expression Mitigates Mutational Escape in Lysis-Driven Bacteria Cancer Therapy. BioDesign Res. 2024, 6. [CrossRef]
[16] Hou, G.; Wu, D.; Li, X.; Liu, B. Tumor Targeted and GSH Stimuli-response α-Lactalbumin Nanotubes co-Delivering Doxorubicin and siRNA for Cancer Therapy. J. Future Foods 2024, 4, 300–308. [CrossRef]
[17] Ma, P.; Wang, G.; Men, K.; Li, C.; Gao, N.; Li, L. Advances in Clinical Application of Nanoparticle-Based Therapy for Cancer Treatment: A Systematic Review. Nano TransMed. 2024, 3, 100036. [CrossRef]
[18] Sharma, R.; Malviya, R. Modifying the Electrical, Optical, and Magnetic Properties of Cancer Cells: A Comprehensive Approach for Cancer Management. Med. Adv. 2024, 2, 3–19. [CrossRef]
[19] Liu, K.; Fang, X.; Aazmi, A.; Wang, Q.; Gong, X.; Chen, Z.; Qin, M.; Pu, C.; Zhao, Y.; Qiu, X.; et al. Organoids: Principle, Application and Perspective. Innov. Life 2024, 2, 100088. [CrossRef]
[20] Gu, A.; Li, J.; Wu, J.-A.; Li, M.-Y.; Liu, Y. Exploration of Dan-Shen-Yin Against Pancreatic Cancer Based on Network Pharmacology Combined with Molecular Docking and Experimental Validation. Curr. Res. Biotechnol. 2024, 7. [CrossRef]
[21] Chen, Y.-H.; Xu, H.; Hu, D.; Xie, C.; Liu, S.-M.; Hu, L.; Xu, D.-L.; Zhao, C.; Yuan, F.-W. Traditional Medicine in Cancer: What is New in 2022. Tradit. Med. Res. 2023, 8, 43–48. [CrossRef]
[22] Wang, Z.; Liu, Z.; Qu, J.; Sun, Y.; Zhou, W. Role of Natural Products in Tumor Therapy from Basic Research and Clinical Perspectives. Acta Mater. Medica 2024, 3, 163–206. [CrossRef]
[23] Yuan, L.; Yang, L.; Zhang, S.; Xu, Z.; Qin, J.; Shi, Y.; Yu, P.; Wang, Y.; Bao, Z.; Xia, Y.; et al. Development of a Tongue Image-Based Machine Learning Tool for the Diagnosis of Gastric Cancer: A Prospective Multicentre Clinical Cohort Study. EClinicalMedicine 2023, 57, 101834. [CrossRef] [PubMed]
[24] Wang, S.-F.; Dong, S.-Q.; Dong, Q.; Lin, W.-X.; Dong, M.; Liu, D. Natural Product-Induced Oxidative Stress-Synergistic Anti-Tumor Effects of Chemotherapeutic Agents. Tradit. Med. Res. 2024, 9, 13–18. [CrossRef]
[25] Feng, T.; Ahmed, W.; Ahmed, T.; Chen, L. Nanoparticles Derived from Herbal Preparations May Represent a Novel Nucleic acid Therapy. Interdiscip. Med. 2024, 2, e20230029. [CrossRef]
[26] Mou, X.; Zhang, A.; He, T.; Chen, R.; Zhou, F.; Yeung, T.C.; Wang, C.-C.; Tang, C.; Lu, X.; Li, L.; et al. Organoid Models for Chinese Herbal Medicine Studies. Acta Mater. Medica 2023, 2, 64–71. [CrossRef]
[27] Shehata, M.M. Anticancer Lipid-Based Drug Delivery Systems: Basic Knowledge and Recent Applications. Nano TransMed 2024, 3, 100054. [CrossRef]
[28] Li, H.; Wang, S.; Zhang, Y.; Li, W. A New Paradigm for Cytology-Based Artificial Intelligence-Assisted Prediction for Cancers of Unknown Primary Origins. Innov. Life 2024, 2, 100086. [CrossRef]
[29] Sun-Waterhouse, D.-X.; Chen, X.-Y.; Liu, Z.-H.; Waterhouse, G.I.N.; Kang, W.-Y. Transformation from Traditional Medicine-Food Homology to Modern Food-Medicine Homology. Food Med. Homol. 2024, 1, 9420014. [CrossRef]
[30] Cong, B. Perspectives in Food & Medicine Homology. Food Med. Homol. 2024, 1, 9420018. [CrossRef]
[31] Li, M.-Y.; Gu, A.; Li, J.; Tang, N.; Matin, M.; Yang, Y.; Zengin, G.; Atanasov, A.G. Exploring Food and Medicine Homology: Potential Implications for Cancer Treatment Innovations. Acta Mater. Medica 2025, 4, 200–206. [CrossRef]
[32] Zhang, X.; Li, S.; Zhang, B.; Zhang, Y. The Structures and Regulatory Roles of Natural Products in Lipid Metabolism: Focus on Medicinal and Edible Plants. Food Sci. Hum. Wellness 2024, . [CrossRef]
[33] Chen, S.; Xin, Y.; Tang, K.; Wu, Y.; Guo, Y. Nardosinone and Aurantio-Obtusin, Two Medicine Food Homology Natural Compounds, Are Anti-Influenza Agents as Indicated by Transcriptome Signature Reversion. Phytomedicine 2023, 108, 154515. [CrossRef] [PubMed]
[34] Niu, H.; Aruhan; Surenjidiin, S.; Zhang, L.-M.; Zhang, C.-H.; Li, M.-H. Yinshan Zhengyao: Exploring the Power of Food and Inheriting Healthy Thoughts. Food Med. Homol. 2024, 1, 9420006. [CrossRef]
[35] Qiang, H.; Xu, T.; Ma, P.; Zhang, S.; Du, G.; Qiang, G.; Ji, T. A Comprehensive Review of Meal Replacement from Dining Table to Sickbed: Beyond Biomedical Potentials. Food Sci. Hum. Wellness 2024, . [CrossRef]
[36] Liu, Z.; Liu, M.; Meng, J.; Wang, L.; Chen, M. A Review of the Interaction Between Diet Composition and Gut Microbiota and Its Impact on Associated Disease. J. Future Foods 2024, 4, 221–232. [CrossRef]
[37] Mu, Z.; Tran, B.-M.; Xu, H.; Yang, Z.; Qamar, U.Z.; Wang, X.; Xiao, Y.; Luo, J. Exploring the Potential Application of Coconut Water in Healthcare and Biotechnology: A Review. Beverage Plant Res. 2024, , 4. [CrossRef]
[38] Gao, J.; Chen, D.; Lin, Z.; Peng, J.; Yu, S.; Zhou, C.; Jiang, H.; Sun, R.; Lin, Z.; Dai, W. Research Progress on the Antidiabetic Activities of Tea and Its Bioactive Components. Beverage Plant Res. 2023, 3. [CrossRef]
[39] Lu, Q.; Li, R.; Yang, Y.; Zhang, Y.; Zhao, Q.; Li, J. Ingredients with Anti-Inflammatory Effect from Medicine Food Homology Plants. Food Chem. 2022, 368, 130610. [CrossRef]
[40] Li, W.; Chen, H.; Xu, B.; Wang, Y.; Zhang, C.; Cao, Y.; Xing, X. Research Progress on Classification, Sources and Functions of Dietary Polyphenols for Prevention and Treatment of Chronic Diseases. J. Future Foods 2023, 3, 289–305. [CrossRef]
[41] Liu, P.; Zhao, H.; Luo, Y. Anti-Aging Implications of Astragalus Membranaceus (Huangqi): A Well-Known Chinese Tonic. Aging Dis. 2017, 8, 868–886. [CrossRef] [PubMed]
[42] Chen, Z.; Liu, L.; Gao, C.; Chen, W.; Vong, C.T.; Yao, P.; Yang, Y.; Li, X.; Tang, X.; Wang, S.; et al. Astragali Radix (Huangqi): A Promising Edible Immunomodulatory Herbal Medicine. J. Ethnopharmacol. 2020, 258, 112895. [CrossRef] [PubMed]
[43] Gao, Z.; Wang, G.; Chen, Y.; Yuan, W.; Cai, J.; Feng, A.; Fang, J.; Xu, Q.; Wu, X. Total Flavonoids of Astragalus Membranaceus Protect Against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Neurotoxicity in Mice by Inhibiting Ferroptosis Through SLC7A11/GPX-4 Signaling Pathway. Food Sci. Hum. Wellness 2024, 13, 414–420. [CrossRef]
[44] Liang, Y.; Xie, Y.; Liu, X.; Yu, L.; Yan, H.; Shang, Z.; Wu, Y.; Cai, X.; Shi, W.; Du, J.; et al. Integrating Network Pharmacology and Experimental Validation to Decipher the Mechanism of Action of Astragalus-Atractylodes Herb Pair in Treating Hepatocellular Carcinoma. Drug Des. Devel Ther. 2024, 18, 2169–2187. [CrossRef]
[45] Lin, Z.; Zhang, Z.; Ye, X.; Zhu, M.; Li, Z.; Chen, Y.; Huang, S. Based on Network Pharmacology and Molecular Docking to Predict the Mechanism of Huangqi in the Treatment of Castration-Resistant Prostate Cancer. PLoS ONE 2022, 17. [CrossRef]
[46] Chu, X.D.; Zhang, Y.R.; Lin, Z.B.; Zhao, Z.; Huangfu, S.C.; Qiu, S.H.; Guo, Y.-G.; Ding, H.; Huang, T.; Chu, X.-L.; et al. A Network Pharmacology Approach for Investigating the Multi-Target Mechanisms of Huangqi in the Treatment of Colorectal Cancer. Transl. Cancer Res. 2021, 10, 681–693. [CrossRef]
[47] Li, L.; Lu, Y.; Liu, Y.; Wang, D.; Duan, L.; Cheng, S.; Liu, G. Network Pharmacology Analysis of Huangqi Jianzhong Tang Targets in Gastric Cancer. Front. Pharmacol. 2022, 13. [CrossRef]
[48] Auyeung, K.K.; Han, Q.B.; Ko, J.K. Astragalus Membranaceus: A Review of its Protection Against Inflammation and Gastrointestinal Cancers. Am. J. Chin. Med. 2016, 44, 1–22. [CrossRef] [PubMed]
[49] Li, Z.; Qi, J.; Guo, T.; Li, J. Research Progress of Astragalus Membranaceus in Treating Peritoneal Metastatic Cancer. J. Ethnopharmacol. 2023, 305, 116086. [CrossRef]
[50] Nogales, C.; Mamdouh, Z.M.; List, M.; Kiel, C.; Casas, A.I.; Schmidt, H. Network Pharmacology: Curing Causal Mechanisms Instead of Treating Symptoms. Trends Pharmacol. Sci. 2022, 43, 136–150. [CrossRef]
[51] Valdés-Tresanco, M.S.; Valdés-Tresanco, E.; Valiente, P.A.; Moreno, E. AMDock: A Versatile Graphical Tool for Assisting Molecular Docking with Autodock Vina and Autodock4. Biol. Direct 2020, 15. [CrossRef]
[52] Kynast, J.P.; Höcker, B. Atligator Web: A Graphical User Interface for Analysis and Design of Protein–Peptide Interactions. BioDesign Res. 2023, 5. [CrossRef] [PubMed]
[53] Zhou, R.; Xu, L.; Ye, M.; Liao, M.; Du, H.; Chen, H. Formononetin Inhibits Migration and Invasion of MDA-MB-231 and 4T1 Breast Cancer Cells by Suppressing MMP-2 and MMP-9 Through PI3K/AKT Signaling Pathways. Horm. Metab. Res. 2014, 46, 753–760. [CrossRef]
[54] Huang, Y.P.; Yeh, C.A.; Ma, Y.S.; Chen, P.Y.; Lai, K.C.; Lien, J.C.; Hsieh, W.-T. PW06 Suppresses Cancer Cell Metastasis in Human Pancreatic Carcinoma MIA PaCa-2 Cells via the Inhibitions of p-Akt/mTOR/NF-κB and MMP2/MMP9 Signaling Pathways In Vitro. Environ. Toxicol. 2024, 39, 2768–2781. [CrossRef] [PubMed]
[55] Ganesan, S.; Faris, A.N.; Comstock, A.T.; Chattoraj, S.S.; Chattoraj, A.; Burgess, J.R.; Curtis, J.L.; Martinez, F.J.; Zick, S.; Hershenson, M.B.; et al. Quercetin Prevents Progression of Disease in Elastase/LPS-Exposed Mice by Negatively Regulating MMP Expression. Respir. Res. 2010, 11, 131. [CrossRef] [PubMed]
[56] Cai, F.; Zhang, Y.; Li, J.; Huang, S.; Gao, R. Isorhamnetin Inhibited the Proliferation and Metastasis of Androgen-Independent Prostate Cancer Cells by Targeting the Mitochondrion-Dependent Intrinsic Apoptotic and PI3K/Akt/mTOR Pathway. Biosci. Rep. 2020, . [CrossRef]
[57] Ju, P.C.; Ho, Y.C.; Chen, P.N.; Lee, H.L.; Lai, S.Y.; Yang, S.F.; Yeh, C.-B. Kaempferol Inhibits the Cell Migration of Human Hepatocellular Carcinoma Cells by Suppressing MMP-9 and Akt Signaling. Environ. Toxicol. 2021, 36, 1981–1989. [CrossRef]
[58] Li, C.; Zhao, Y.; Yang, D.; Yu, Y.; Guo, H.; Zhao, Z.; Zhang, B.; Yin, X. Inhibitory Effects Of Kaempferol on the Invasion of Human Breast Carcinoma Cells by Downregulating the Expression and Activity of Matrix Metalloproteinase-9. Biochem. Cell Biol. 2015, 93, 16–27. [CrossRef]
[59] Li, J.; Gu, A.; Li, M.Y. Heteroaryl Group Containing Trisubstituted Alkenes: Synthesis and Anti-Tumor Activity. Chem. Biodivers. 2024, 21. [CrossRef]
[60] Gu, A.; Li, J.; Qiu, S.; Hao, S.; Yue, Z.Y.; Zhai, S.; Li, M.-Y.; Liu, Y. Pancreatic Cancer Environment: From Patient-Derived Models to Single-Cell Omics. Mol. Omics. 2024, 20, 220–233. [CrossRef]
[61] Hu, Y.; Zeng, N.; Ge, Y.; Wang, D.; Qin, X.; Zhang, W.; Jiang, F.; Liu, Y. Identification of the Shared Gene Signatures and Biological Mechanism in Type 2 Diabetes and Pancreatic Cancer. Front. Endocrinol (Lausanne). 2022, 13. [CrossRef] [PubMed]
[62] Padoan, A.; Plebani, M.; Basso, D. Inflammation and Pancreatic Cancer: Focus on Metabolism, Cytokines, and Immunity. Int. J. Mol. Sci. 2019, . [CrossRef] [PubMed]
[63] Brayer, K.J.; Hanson, J.A.; Cingam, S.; Martinez, C.; Ness, S.A.; Rabinowitz, I. The inflammatory Response of Human Pancreatic Cancer Samples Compared to Normal Controls. PLoS ONE 2023, 18. [CrossRef]
[64] Gardi, C.; Bauerova, K.; Stringa, B.; Kuncirova, V.; Slovak, L.; Ponist, S.; Drafi, F.; Bezakova, L.; Tedesco, I.; Acquaviva, A.; et al. Quercetin Reduced Inflammation and Increased Antioxidant Defense in Rat Adjuvant Arthritis. Arch. Biochem. Biophys. 2015, 583, 150–157. [CrossRef] [PubMed]
[65] Lu, X.; Ye, Z.; Jiang, L.; Wen, L.; Liu, J.; Cui, Q. UPLC-Q-TOF-MS/MS Analysis of Pomegranate Peel Polyphenols and Preliminary Study on the Biosynthesis of Ellagic Acid and Flavonoids. Beverage Plant Res. 2024, 4. [CrossRef]
[66] Mohammed, Y.H.I.; Shamkh, I.M.; Alharthi, N.S.; Shanawaz, M.A.; Alzahrani, H.A.; Jabbar, B.; Beigh, S.; Alghamdi, S.; Alsakhen, N.; Khidir, E.B.; et al. Discovery of 1-(5-bromopyrazin-2-yl)-1-[3-(trifluoromethyl)benzyl]urea as a Promising Anticancer Drug via Synthesis, Characterization, Biological Screening, and Computational Studies. Sci. Rep. 2023, 13. [CrossRef]
[67] Rabie, A.M.; Tantawy, A.S.; Badr, S.M.I. Design, Synthesis, and Biological Evaluation of New 5-Substituted-1,3,4-thiadiazole-2-thiols as Potent Antioxidants. Researcher 2018, 10, 21–43. [CrossRef]
[68] Zizkova, P.; Stefek, M.; Rackova, L.; Prnova, M.; Horakova, L. Novel Quercetin Derivatives: From Redox Properties to Promising Treatment of Oxidative Stress Related Diseases. Chem.-Biol. Interact. 2017, 265, 36–46. [CrossRef]
[69] Wang, J.-L.; Quan, Q.; Ji, R.; Guo, X.-Y.; Zhang, J.-M.; Li, X.; Liu, Y.-G. Isorhamnetin Suppresses PANC-1 Pancreatic Cancer Cell Proliferation Through S Phase Arrest. Biomed. Pharmacother. 2018, 108, 925–933. [CrossRef]
[70] Han, F.; Dong, S.; Chen, Z.; Dong, C.; Shi, H.; Du, Y.; Zhou, W. Ciprofol Suppresses Proliferation, Invasion and Migration of Human Pancreatic Cancer Cells. Pak. J. Pharm. Sci. 2024, 37, 327–336.
[71] Shen, X.; Si, Y.; Wang, Z.; Wang, J.; Guo, Y.; Zhang, X. Quercetin Inhibits the Growth of Human Gastric Cancer Stem Cells by Inducing Mitochondrial-Dependent Apoptosis Through the Inhibition of PI3K/Akt Signaling. Int. J. Mol. Med. 2016, 38, 619–626. [CrossRef] [PubMed]
[72] Wang, F.; Wang, L.; Qu, C.; Chen, L.; Geng, Y.; Cheng, C.; Yu, S.; Wang, D.; Yang, L.; Meng, Z.; et al. Kaempferol Induces ROS-Dependent Apoptosis in Pancreatic Cancer Cells via TGM2-Mediated Akt/mTOR Signaling. BMC Cancer 2021, 21. [CrossRef] [PubMed]
[73] Liu, Z.Q.; Yao, G.L.; Zhai, J.M.; Hu, D.W.; Fan, Y.G. Kaempferol Suppresses Proliferation and Induces Apoptosis and DNA Damage in Human Gallbladder Cancer Cells Through the CDK4/CDK6/cyclin D1 Pathway. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1311–1321. [CrossRef]
[74] Ge, Y.; Zhang, Y.; Chen, Y.; Li, Q.; Chen, J.; Dong, Y.; Shi, W. Silibinin Causes Apoptosis and Cell Cycle Arrest in Some Human Pancreatic Cancer Cells. Int. J. Mol. Sci. 2011, 12, 4861–4871. [CrossRef]
[75] Johnson, J.L.; Gonzalez de Mejia, E. Interactions Between Dietary Flavonoids Apigenin or Luteolin and Chemotherapeutic Drugs to Potentiate Anti-Proliferative Effect on Human Pancreatic Cancer Cells, in vitro. Food Chem. Toxicol. 2013, 60, 83–91. [CrossRef] [PubMed]
[76] Vendrely, V.; Peuchant, E.; Buscail, E.; Moranvillier, I.; Rousseau, B.; Bedel, A.; Brillac, A.; de Verneuil, H.; Moreau-Gaudry, F.; Dabernat, S. Resveratrol and Capsaicin Used Together as Food Complements Reduce Tumor Growth and Rescue Full Efficiency of Low Dose Gemcitabine in a Pancreatic Cancer Model. Cancer Lett. 2017, 390, 91–102. [CrossRef]
[77] Wang, H.; Li, Q.F.; Chow, H.Y.; Choi, S.C.; Leung, Y.C. Arginine Deprivation Inhibits Pancreatic Cancer Cell Migration, Invasion and EMT via the Down Regulation of Snail, Slug, Twist, and MMP1/9. J. Physiol. Biochem. 2020, 76, 73–83. [CrossRef]
[78] Su, M.; Miao, F.; Jiang, S.; Shi, Y.; Luo, L.; He, X.; Wang, J.; Xu, S.; Lei, T.-C. Role of the p53-TRPM1/miR-211-MMP9 Axis in UVB-Induced Human Melanocyte Migration and Its Potential in Repigmentation. Int. J. Mol. Med. 2020, 45, 1017–1026. [CrossRef]
[79] Zhao, M.; Tang, S.N.; Marsh, J.L.; Shankar, S.; Srivastava, R.K. Ellagic Acid Inhibits Human Pancreatic Cancer Growth in Balb c Nude Mice. Cancer Lett. 2013, 337, 210–217. [CrossRef]
[80] Bloomston, M.; Zervos, E.E.; Rosemurgy, A.S. Matrix Metalloproteinases and Their Role in Pancreatic Cancer: A Review of Preclinical Studies and Clinical Trials. Ann. Surg. Oncol. 2002, 9, 668–674. [CrossRef]
[81] Zhang, L.; Wu, G.; Herrle, F.; Niedergethmann, M.; Keese, M. Single Nucleotide Polymorphisms of Genes for EGF, TGF-β and TNF-α in Patients with Pancreatic Carcinoma. Cancer Genom. Proteom. 2012, 9, 287–295.
[82] Kaur, A.; Riaz, M.S.; Singh, S.K.; Kishore, U. Human Surfactant Protein D Suppresses Epithelial-to-Mesenchymal Transition in Pancreatic Cancer Cells by Downregulating TGF-β. Front. Immunol. 2018, 9. [CrossRef] [PubMed]
[83] Kochumon, S.; Al-Sayyar, A.; Jacob, T.; Bahman, F.; Akhter, N.; Wilson, A.; Sindhu, S.; Hannun, Y.A.; Ahmad, R.; Al-Mulla, F. TGF-β and TNF-α Interaction Promotes the Expression of MMP-9 through H3K36 Dimethylation: Implications in Breast Cancer Metastasis. Front. Immunol. 2024, 15. [CrossRef] [PubMed]
We use cookies to improve your experience on our site. By continuing to use our site, you accept our use of cookies. Learn more