
APA Style
Saad Ahmed, Muhammad Shayaan Mazhar, Muhammad Fahad Shabbir. (2024). Neoantigen-Based Cancer Vaccines: Current Innovations, Challenges and Future Directions in Personalized Immunotherapy. Cancer Immunology Connect, 1 (Article ID: 0001). https://doi.org/10.69709/CIConnect.2024.194763MLA Style
Saad Ahmed, Muhammad Shayaan Mazhar, Muhammad Fahad Shabbir. "Neoantigen-Based Cancer Vaccines: Current Innovations, Challenges and Future Directions in Personalized Immunotherapy". Cancer Immunology Connect, vol. 1, 2024, Article ID: 0001, https://doi.org/10.69709/CIConnect.2024.194763.Chicago Style
Saad Ahmed, Muhammad Shayaan Mazhar, Muhammad Fahad Shabbir. 2024. "Neoantigen-Based Cancer Vaccines: Current Innovations, Challenges and Future Directions in Personalized Immunotherapy." Cancer Immunology Connect 1 (2024): 0001. https://doi.org/10.69709/CIConnect.2024.194763.
 ACCESS
Review Article
ACCESS
Review Article
Volume 1, Article ID: 2024.0001

Saad Ahmed
saad0ahmed18@gmail.com

Muhammad Shayaan Mazhar
shayaanmazhar7@gmail.com

Muhammad Fahad Shabbir
fahadshabbir166@gmail.com
1 Department of Biological Sciences, International Islamic University Islamabad, Islamabad 44000, Pakistan
2 Department of Biotechnology, Quaid-I-Azam University, Islamabad 45320, Pakistan
* Author to whom correspondence should be addressed
Received: 30 Aug 2024 Accepted: 14 Oct 2024 Available Online: 16 Oct 2024 Published: 21 Oct 2024
The development of neoantigen-based cancer vaccines represents a breakthrough in personalized immunotherapy due to their ability to elicit strong and effective tumor-specific immune responses. Unlike conventional cancer vaccines, neoantigen vaccines target mutations found explicitly in tumor cells, thus maximizing specificity and minimizing immune tolerance. The review explores the current status of neoantigen vaccines and their mechanisms, development, and clinical applications. In contrast, it also explores the challenges of personalized vaccines, such as identifying neoantigens and their manufacturing, along with biological factors and regulatory hurdles. Future innovations and strategies are discussed to overcome resistance and relapse. Critical issues like global access, equity, and scalability must be addressed for its more comprehensive implementation. This review article discusses the potential of neoantigen vaccines in cancer therapy, detailing ongoing research and clinical trials, while addressing the persistent challenges that must not be underestimated.
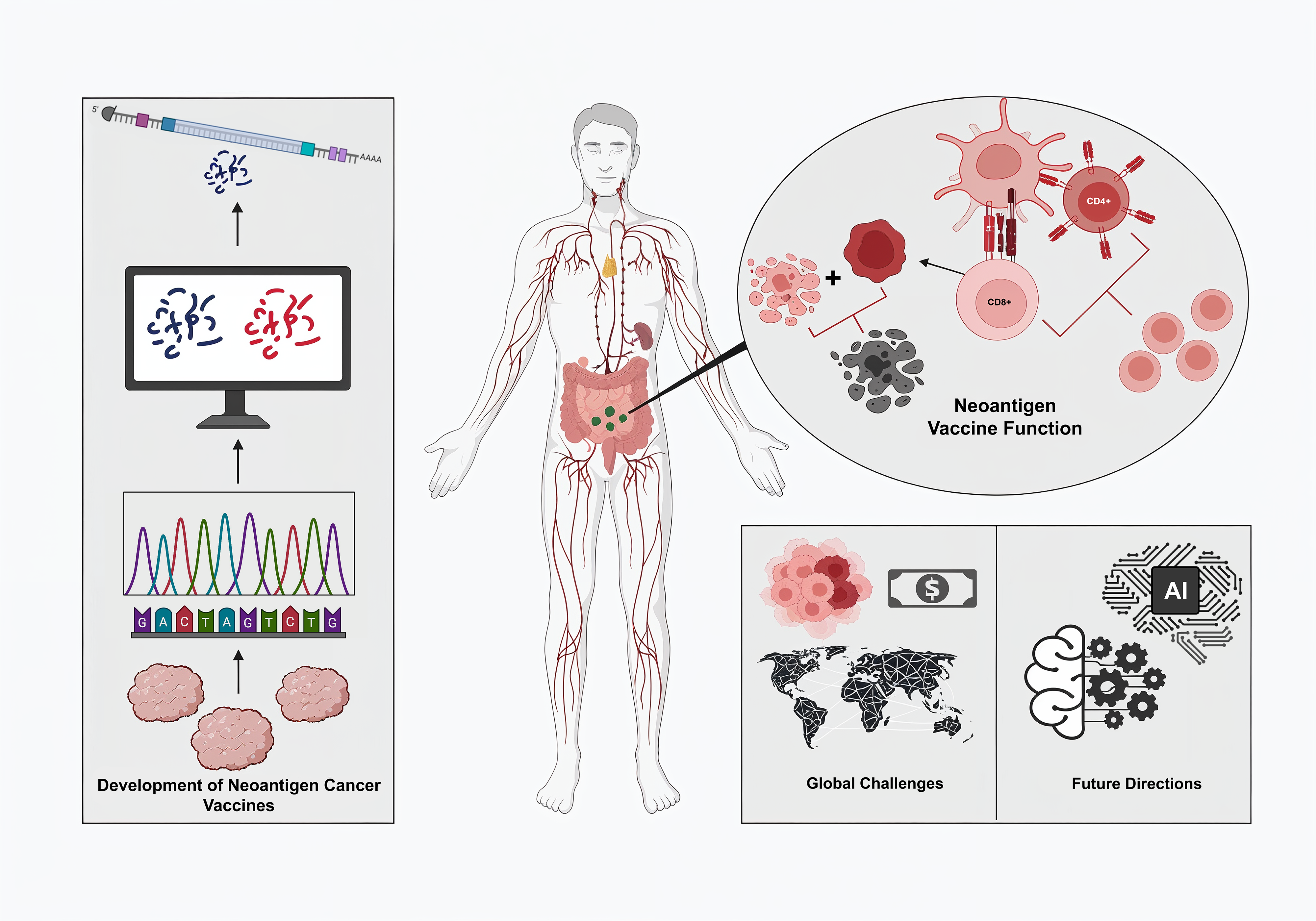
Cancer, one of the leading causes of mortality worldwide, accounts for millions of deaths annually. Despite advances in surgery, chemotherapy, and radiation therapy, traditional therapeutic approaches are generally inadequate for achieving long-term control of most cancers, particularly at the advanced levels of cancer [1]. Recently, immunotherapy has emerged as a new approach to cancer treatment by utilizing the body’s immune system to recognize and destroy malignant cells. Among the various immunotherapy applications, cancer vaccines have gained the limelight due to their potential to evoke specific immune responses against tumors [2]. Cancer vaccines stimulate the immune system to attack cancer cells. The fundamental principle of cancer vaccines is to identify molecules that can trigger an immune response unique to cancer cells [3]. In contrast, the early development of cancer vaccines focused on tumor-associated antigens (TAAs), such as carcinoembryonic antigen (CEA) in colorectal cancer and prostate-specific antigen (PSA) in prostate cancer, as both proteins are expressed at a higher level in cancer cells than in normal cells. These vaccines aim to provoke an immune response by presenting these antigens to the immune system, thereby marking cancer cells for destruction [4]. However, there were challenges that TAA-based vaccines face, which eventually leads to limit their efficacies. One key limitation was tumor antigen heterogeneity, where cancers consist of diverse cell populations with different antigenic profiles, allowing some cancer cells to escape immune detection if they do not express the targeted TAAs. Since TAAs are often self-antigens present at low levels in normal tissues, the immune system develops tolerance, which leads to an inadequate response. These challenges underscore the necessity for more personalized and precise cancer vaccines. [5]. Advancements in bioinformatics, such as next-generation sequencing (NGS), have made it possible to discover novel peptides of neoantigens that arise from somatic mutations specific to tumor cells [6]. TAAs, on the other hand, are not found in normal tissues; therefore, neoantigens offer an ideal target for cancer vaccines. This significant evolution allows the development of personalized therapies that can overcome the limitations of traditional approaches. Neoantigen vaccines are designed to induce powerful T-cell responses against such unique, tumor-specific antigens, offering a more effective option for treatment in cancer patients [7]. Neoantigens have revolutionized cancer immunotherapy into a truly personalized treatment method. These antigens are unique to each patient’s cancer since they are derived from nonsynonymous mutations in the tumor’s genome, making them highly specific targets for immune recognition. Unlike TAAs, which are often shared across different individuals and types of cancers, neoantigens are patient-specific and recognized as foreign by the immune system, preventing immune tolerance. The neoantigen-based vaccines are personalized treatments that use the DNA sequence of a patient’s tumor to identify mutations capable of encoding neoantigens. The selection must be based on the neoantigen’s ability to bind to the patient’s major histocompatibility complex (MHC), which is crucial for effective T cell recognition. The selected neoantigens are then synthesized and formulated into a vaccine, eliciting a strong and targeted immune response toward the tumor [9]. Clinical trials have demonstrated that neoantigen vaccines hold a significant promise for different cancers that have not responded to conventional therapies. For example, patients with melanoma responded strongly, and immune responses were maintained up to four years post-treatment, while some remained free from disease. This long-term effect occurs because the vaccine can activate T cells that target tumor-specific antigens, thus controlling growth to prevent the tumor from reoccurring [10]. This review explores the current landscape in neoantigen cancer vaccines, focusing on their evolution, immune mechanisms, and integration into therapies, including immune checkpoint inhibitors. Subsequently, it discusses technological, biological, and clinical challenges associated with the role of AI in vaccine design, as well as ethical issues related to access and equity in personalized therapies. Future research directions address overcoming resistance, improving platforms, and extending global access.
The literature review employed a narrative approach to summarize and analyze cancer vaccine research, focusing on neoantigen-based immunotherapy. Databases like PubMed, Google Scholar, and Web of Science were searched using keywords such as “cancer vaccines” and “neoantigens”. Peer-reviewed articles, clinical trials, and research papers from 2015–2024 providing significant insights into cancer vaccine development and clinical applications were prioritized. Studies lacking relevance, rigorous peer review, or duplicative were excluded. The selection process included screening titles, abstracts, and full texts regarding key data derived mostly from clinical trials. While the narrative approach tends to give a more comprehensive overview of emerging trends and challenges with cancer immunotherapy, potential biases were addressed by including older studies to capture the most cutting-edge information.
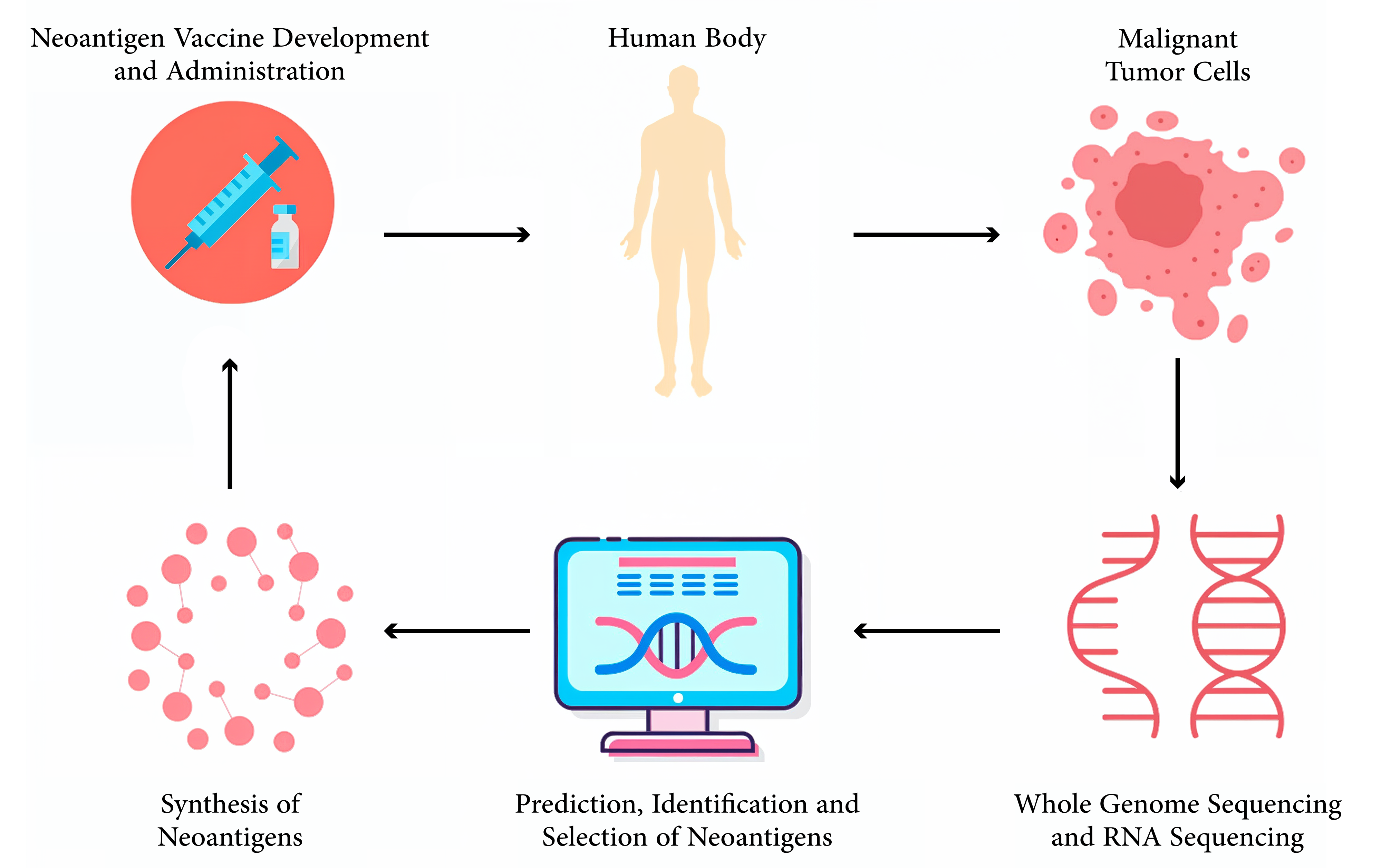
Over the past few decades, there has been significant progress in cancer vaccines, particularly with the recent advancements in tumor immunology integrated with modern technology. Today, cancer vaccines have not been confined to traditional methods but rather extended to highly personalized approaches, like neoantigen-based vaccines (Table 1). Generally, conventional vaccines against cancer are divided into two: prophylactic and therapeutic, with each specific role directed at preventing and treating cancer. Prophylactic vaccines are designed to prevent cancers associated with infectious agents, such as viruses, by targeting them before malignant transformation. Therapeutic vaccines aim at treating existing cancers by prompting the immune system to recognize and attack tumor cells. The development of prophylactic cancer vaccines has been successful and promising in preventing virus-associated malignancies. For example, the human papillomavirus (HPV) vaccines Gardasil and Cervarix have decreased occurrences of cervical cancer through a focus on the high-risk HPV strains responsible for 90% of cases. These vaccines produce neutralizing antibodies that prevent viral infection and subsequent malignant transformation [11]. The hepatitis B virus (HBV) vaccine has also reduced the incidence of HBV-related hepatocellular carcinoma through the prevention of chronic HBV infection, which is considered a major risk factor for liver cancer [12]. Therapeutic cancer vaccines, on the other hand, are designed to treat existing cancers by stimulating an immune response to TAAs [5]. An example is sipuleucel-T (Provenge), approved by the Food and Drug Administration (FDA) for the treatment of metastatic castration-resistant prostate cancer. Sipuleucel-T is an autologous cellular immunotherapy utilizing a fusion protein of prostatic acid phosphatase (PAP) with granulocyte-macrophage colony-stimulating factor (GM-CSF), applied to the patient’s dendritic cells. This results in the activation of the dendritic cells to stimulate a PAP-targeted immune response, an antigen expressed in most prostate cancer cells [13]. Although these vaccines have been successful, traditional approaches still face significant challenges in addressing the heterogeneity of tumor antigens. Tumors are composed of several cell populations expressing different profiles of antigens. A vaccine targeting only one or a few TAAs might fail to eradicate all cancer cells since there is a lack of targeted antigens that allow the escape of an immune response and continuous proliferation [14]. In addition, tumors have developed different strategies of immune evasion, like genetic heterogeneity, antigen expression downregulation, alteration in the pathways of antigen presentation, and development of an immune suppressive microenvironment, which counter the traditional vaccines [9]. The risk of immune tolerance is a significant hurdle for therapeutic vaccines, since most TAAs are the overexpressed normal self-antigens. As a result, the immune system may recognize these antigens as self and fail to generate a sufficiently strong response, further limiting the effectiveness of conventional vaccines. In this regard, the above complexity and dynamism in the biology of tumors signify that there is a clamor for sophisticated personalized approaches [15]. The advent of neoantigen-based vaccines marks a significant leap forward in cancer immunotherapy. Neoantigens are the class of antigens whose specificity lies with the tumor, originating from somatic mutations specific to an individual’s cancer. In contrast, TAAs are often overexpressed proteins in normal and cancerous tissues but are not novel to the tumor. This distinction gives neoantigens high immunogenicity, making them excellent targets for personalized cancer vaccines. [7]. Neoantigens are usually derived from non-synonymous mutations that result in the alteration of amino acid sequences of proteins within tumor cells. These mutant proteins can be processed by antigen-presenting cells and presented by MHC molecules on the surface of cancer cells, from which T cells recognize them as foreign substances [16]. Neoantigens, found exclusively in the tumor and completely lacking in normal tissue, do not undergo immune-tolerance mechanisms. Instead, they help balance the immune response to TAAs, promoting a more vigorous and coordinated attack on cancer cells [17]. Traditional Vaccines vs Neoantigen- Based Vaccines. Neoantigen-based vaccine development is technology-intensive and dependent on discoveries in genomics and bioinformatics. This is driven by next-generation sequencing, which enables a comprehensive analysis of the genetic structure of a tumor. Whole-exome sequencing (WES) is commonly used to capture non-synonymous mutations in gene-coding regions. Those mutated genes are then put through intricate bioinformatics tools to predict which mutations might give rise to peptides that can bind to the patient’s MHC molecules, a critical determinant of their potential as neoantigens. Integrating artificial intelligence and machine learning into this process has further enhanced the accuracy and efficiency of neoantigen prediction. AI-driven methods for the processing of large volumes of genomic data are used to recognize patterns and, in turn, enhance the prediction of neoantigens that are likely to trigger a strong immune response. Moreover, such approaches would also be applied in vaccine design optimization to predict ideal neoantigen combinations that maximize the therapeutic efficacy of a vaccine (Figure 1) [18,19]. Clinical trials of neoantigen-based vaccines have shown promising results, particularly in cancers with high mutational burdens, such as melanoma and non-small cell lung cancer (NSCLC). Currently, neoantigen-based vaccines are under evaluation in several clinical trials for patients with different types of cancers; some are summarized in Table 2. These span from Phase I to Phase II trials and cover various vaccine formulations, from DNA and mRNA to peptides, often with additional interventions, including checkpoint inhibitors and chemotherapy. Neoantigen-Based Vaccine Clinical Trials. Despite these advancements, neoantigen vaccines face various challenges. The technical and logistical complexities of neoantigen identification and vaccine production become a huge obstacle to clinical implementation. In addition, the high cost of developing personalized vaccines can raise concerns about access and equity in cancer treatment. Ongoing research aims to overcome current challenges in designing and delivering improved neoantigen vaccines, while also exploring combinations with other immunotherapeutic strategies to achieve maximal efficacy.
Characteristics
Traditional Cancer Vaccines
Neoantigen-Based Vaccines
Antigen Source
TAAs often overexpressed self-antigens
Neoantigens, derived from somatic
mutations unique to the individual’s tumor
Immune Response
Risk of immune tolerance due to antigen similarity with normal cells
Highly immunogenic, with a strong and specific immune response against tumor cells
Tumor Specificity
Limited, as TAAs may be present in both normal and cancerous tissues
High specificity, as neoantigens are unique to tumor cells
Effectiveness
Variable, often limited by tumor heterogeneity and immune evasion
Potentially more effective due to personalized targeting of tumor-specific antigens
Development Complexity
Less complex, based on known TAAs
Highly complex, requiring advanced genomic analysis and bioinformatics
Cost and Accessibility
Generally lower cost, but limited effectiveness in some cases
Higher cost due to personalized nature, with challenges in widespread accessibility
Examples
HBV vaccine, sipuleucel-T (Provenge)
Personalized neoantigen vaccines in clinical trials for melanoma, glioblastoma
Clinical Trail ID
Country
Phase
Cancer Type
Intervention
Status
NCT05475106
Mexico
I
Neoplasms
Neoantigen
PeptidesRecruiting
NCT01970358
United
StatesI
Melanoma
Poly-ICLC,
PeptidesCompleted
NCT05749627
China
N/A
Solid Tumors
Neoantigen
PeptidesRecruiting
NCT06341907
China
II/III
Ovarian Cancer
Neoantigen
PolypeptideRecruiting
NCT05743595
United
StatesI
Glioblastoma
Retifanlimab,
DNA VaccineRecruiting
NCT04509167
Mexico
I
Neoplasms
Neoantigen
PeptidesCompleted
NCT04015700
United
StatesI
Glioblastoma
DNA Vaccine
Active
NCT04397003
United States
II
Extensive- Stage Small Cell Lung
CancerDurvalumab, DNA Vaccine
Recruiting
NCT03532217
United
StatesI
Prostate Cancer
Nivolumab
Completed
NCT03988283
United
StatesI
Brain Tumor
Personalized
DNA VaccineRecruiting
NCT05354323
Lithuania
I
Solid Tumor
DNA Vaccine
Recruiting
NCT04251117
United States/
New ZealandI/II
Hepatocellular Carcinoma
Pembrolizumab
Active
Neoantigen-based vaccines have marked a transformative step in cancer immunotherapy. The foundation of neoantigen vaccine efficacy lies in their capacity to induce a potent and lasting immune response, which involves intricate molecular and cellular mechanisms. The function of neoantigen vaccines is based on the concept of antigen specificity. The activation of the immune response following neoantigen vaccination begins with the uptake of the vaccine by antigen-presenting cells, particularly dendritic cells. These cells play a pivotal role in processing and presenting antigens to T cells, which is a crucial step for initiating an immune response. Upon internalizing the neoantigens, dendritic cells process them into peptide fragments and display these fragments on their surface via MHC molecules. The type of MHC molecule involved, either class I or class II, determines the pathway of T cell activation [20]. Neoantigens on MHC class I molecules are recognized by CD8+ T cells, leading to the activation of cytotoxic T lymphocytes (CTLs). These CTLs are directly responsible for identifying and destroying tumor cells expressing the specific neoantigen. Conversely, neoantigens presented on MHC class II molecules are recognized by CD4+ T helper cells. These helper cells play a crucial role in amplifying the immune response, primarily by secreting cytokines that enhance the function of CTLs and recruit other immune cells [21]. The immune response’s specificity is further sharpened by the clonal expansion of T cells that recognize the neoantigen. Upon activation, these T cells undergo rapid proliferation and differentiate into effector cells capable of targeting and eradicating tumor cells. This proliferation is essential for generating a sufficient number of immune cells to mount an effective attack against the cancer and inhibit tumor progression [22]. The primary objective of neoantigen-based vaccines is to facilitate the destruction of tumor cells. Once CTLs reach the tumor site, they recognize cancer cells presenting the neoantigens. The killing of these tumor cells is mediated by the release of cytotoxic molecules, such as perforin and granzymes, which induce apoptosis, a form of programmed cell death in the target cells. This process not only reduces the tumor burden but also releases additional tumor antigens, further stimulating the immune system through a process known as epitope spreading [23]. A critical aspect of neoantigen-based immunotherapy is the establishment of immune memory. After the initial immune response, some T cells differentiate into memory T cells that persist long after the vaccine has been administered. These memory T cells provide long-term protection by rapidly responding to any tumor recurrence, thus reducing the risk of relapse and offering sustained tumor control (Figure 2) [24]. Clinical trials have demonstrated that patients vaccinated with neoantigens, particularly those with melanoma, can develop long-lasting memory T-cell responses. For instance, in melanoma, who received personalized neoantigen vaccines showed persistent memory T cell responses and epitope spreading over several years [10]. This leads to suggest that the immune system retains memory of the targeted neoantigens and expands its response to other tumor-associated antigens, eventually improving the likelihood of long-term tumor control. The success of neoantigen vaccines is due to immune activation mechanisms and the neoantigens’ immunogenicity. Several factors influence the ability of neoantigens to elicit a robust immune response (Table 3). Factors Influencing the Efficacy of Neoantigens.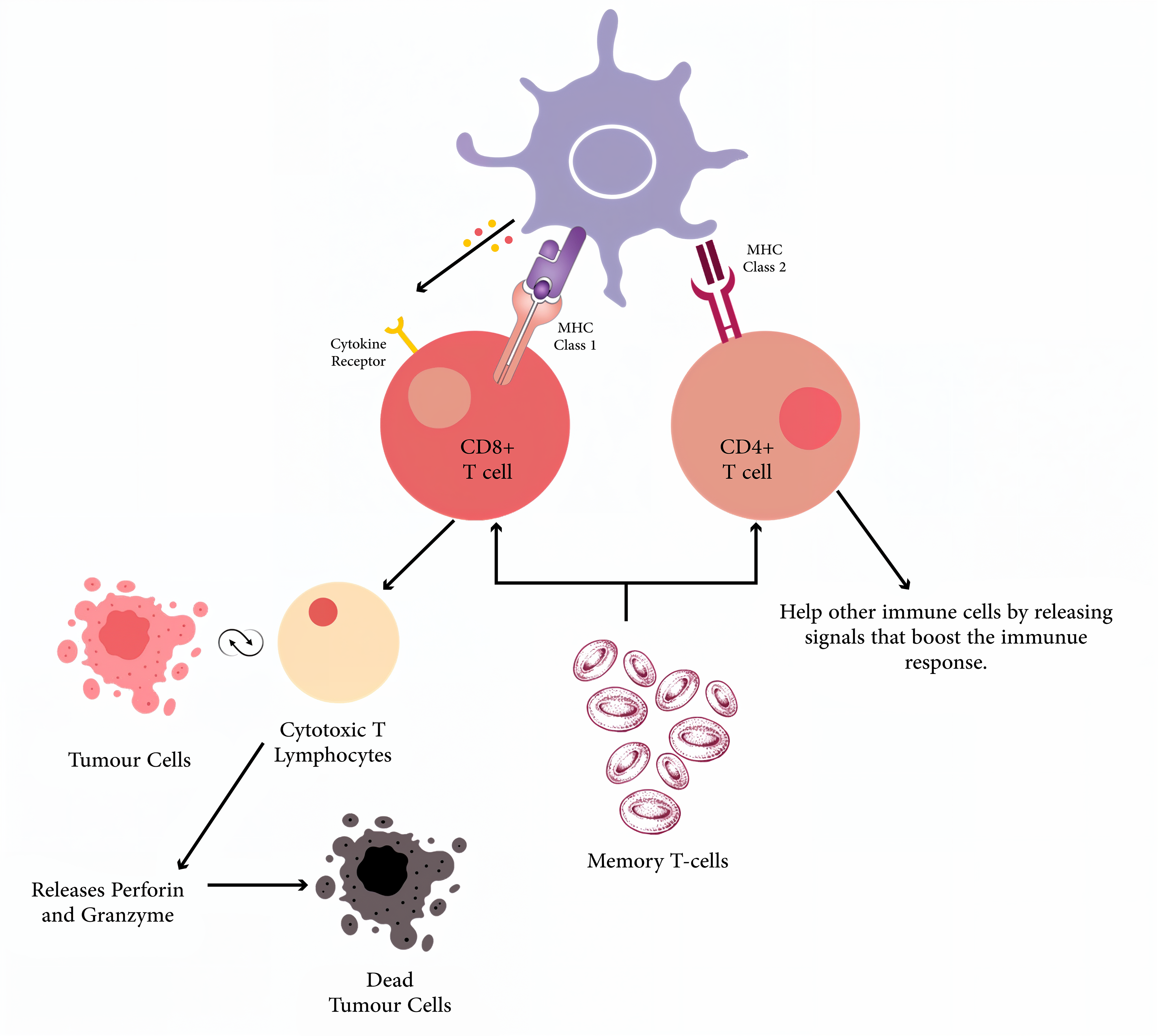
Factor
Description
Impact on Neoantigen
Vaccines
Tumor Mutation Burden
[25]The number of mutations present in a tumor. High TMB leads to a greater
number of neoantigens.Tumors with high TMB are more likely to generate immunogenic neoantigens,
enhancing vaccine efficacy.
Binding Affinity for MHC Molecules
[26]The strength with which neoantigens bind to MHC molecules for presentation
to T cells.Higher binding affinity improves neoantigen presentation and increases
immunogenicity.
Tumor Microenvironment
[27]The local environment surrounding the tumor, including regulatory T cells (Tregs) and myeloid-derived
suppressor cells (MDSCs).An immunosuppressive TME can hinder vaccine efficacy; strategies to modify the TME are
required.
Bioinformatics and Machine Learning
[28]Techniques used to predict neoantigen-MHC binding and select promising vaccine
targets.Enhances the selection of neoantigens with high immunogenic potential for
vaccine development.
Immune Checkpoint Inhibitor
[29]Checkpoint inhibitors block signals that suppress T cell activity, enhancing immune response.
Synergistic effect with neoantigen vaccines; improves specificity and efficacy of the immune
response.
Use of Adjuvants
[30]Substances that enhance the effectiveness of the vaccine, such as those that improve
dendritic cell function.Improves the immune response by boosting antigen presentation and T
cell activation.
Neoantigen-based vaccines are the most promising in cancer immunotherapy, as they mobilize the immune system with specificities against the tumor cells. Nevertheless, with the promise come many challenges that must be addressed for successful development and implementation. One of the fundamental technical challenges is accurately identifying and selecting the neoantigens in highly heterogeneous tumors. Tumor cells exhibit significant variability, meaning neoantigens in some cells may be absent in others, resulting in incomplete vaccine targeting [31]. Advanced bioinformatics approaches for predicting immunogenic neoantigens are often error-prone and computationally intensive, requiring significant expertise [32]. While next-generation sequencing can analyze the exome and transcriptome, only a fraction of the data is relevant for vaccine design, complicating the prediction of peptides capable of binding to MHC molecules and eliciting a potent T-cell response [24,33]. Moreover, the evolving mutation landscape of tumors can affect vaccine efficacy over time [9]. Despite the promising potential of neoantigen vaccines, resistance development remains a significant concern. Cancer cells can evolve mechanisms to evade immune recognition, reducing vaccine efficacy over time [35]. Some tumors may lose or alter the expression of neoantigens, making them less visible to T cells, while others can downregulate MHC molecules, impeding the presentation of neoantigens and thus escaping detection by cytotoxic T lymphocytes. Additionally, the immunosuppressive tumor microenvironment (TME) often inhibits the activity of memory T cells, thereby compromising the maintenance of long-term immunity [36]. These challenges necessitate combination therapies, such as pairing neoantigen vaccines with immune checkpoint inhibitors (e.g., PD-1/PD-L1 inhibitors), to enhance the immune response and counteract the suppressive effects of the TME. Although platforms such as DNA, mRNA, and synthetic peptide vaccines have shown promise as cost-effective strategies, consistently demonstrating their efficacy in cancer therapy has been challenging. Clinical trial data reveal varying therapeutic outcomes. For instance, a phase-1 trial involving the personalized neoantigen vaccine, NeoVax, administered to eight patients with stage IIIB/C or IVM1b melanoma, showed that all patients developed persistent T-cell responses post-vaccination. However, only 75% (6 out of 8 patients) remained disease-free over a median follow-up period of four years, suggesting that while the vaccine could induce a durable immune response, it did not guarantee complete tumor control [10]. Moreover, a phase 3 clinical trial, in which 745 patients (405 with minimal residual disease and 338 with significant residual disease) were assigned to receive rindopepimut with temozolomide (n = 371) or control with temozolomide (n = 374). The study was stopped early due to futility, showing no significant difference in overall survival for MRD patients. Common serious side effects included thrombocytopenia (9% vs. 6%), fatigue (2% vs. 5%), and brain edema (2% vs. 3%). Sixteen deaths occurred due to side effects, with one potentially linked to rindopepimut. [54]. Similarly, a phase 1/2 study on an mRNA-based neoantigen vaccine in non-small cell lung cancer (NSCLC) indicated that although 57% (8 out of 14) of patients developed a significant T-cell response, only 14% (2 out of 14) experienced objective tumor shrinkage, highlighting the limitations of the vaccine’s anti-tumor effects [35]. Another trial targeting advanced glioblastoma patients with synthetic peptide vaccines demonstrated that only 20% (3 out of 15) exhibited a significant immune response, with just one patient achieving partial tumor regression [35]. These outcomes suggest that while neoantigen vaccines can activate the immune system, translating this activation into durable clinical outcomes such as prolonged survival or complete tumor regression is not always consistent. The variability in response rates emphasizes the need for combination therapies to improve efficacy. A recent study showed that combining a neoantigen vaccine with an immune checkpoint inhibitor (anti-PD-1 therapy) significantly increased efficacy, with around 55% of patients achieving an objective response compared to only 20% with the vaccine alone [40]. This finding underscores the potential of combinatorial approaches to enhance the effectiveness of neoantigen vaccines, making them more reliable in achieving sustained anti-tumor responses. Another technical challenge would be the logistical and manufacturing complexity of producing personalized vaccines. From this perspective, the production of neoantigen vaccines is from conventional vaccines, which are supposed to be mass-produced. The synthesis of peptides or nucleic acids of identified neoantigens is a patient-specific process and, by definition, time-consuming and expensive [8]. It comprises steps such as sequencing, neoantigen prediction, peptide synthesis, and vaccine formulation, each of which should take place within a very small timeframe so that the vaccine remains relevant to the neoantigen profile of the tumor [34]. These challenges are further exacerbated by scalability issues, as such bespoke therapies can only be manufactured in dedicated facilities by trained personnel. This limits their accessibility, particularly in resource-constrained settings. [35]. Apart from being the technical stumbling block, significant biological hurdles stand out and help drive neoantigen vaccine development. Tumor immune escape remains a major concern as cancer cells evolve ways of escaping immune detection and destruction. One such mechanism includes antigen loss in the case of tumor cells, where they downregulate or lose the expression of targeted neoantigens completely [36]. This can be due to genetic changes that lead to the loss of the neoantigen or changes in the machinery of antigens’ presentation, like the loss of MHC molecules. All vaccine benefits are lost if antigen loss occurs, as the immune system cannot identify and target the tumor cells [37]. The plot thickens with the induction of a tumor immune tolerance microenvironment. Tumors recruit immune-suppressive cells such as regulatory T cells, myeloid-derived suppressor cells (MDSCs), and tumor-associated macrophages (TAMs) that inhibit the activity of effector T cells and other immune cells [38]. Since these immunosuppressive cells exist in the presence of immune checkpoint molecules like PD-L1, an unfavorable environment for the activity of vaccine-induced T cells would be created [39]. To overcome this challenge, combinatorial regimens containing immune checkpoint inhibitors or other agents that modulate the TME are now being tested for their ability to enhance neoantigen vaccine efficacy [40]. Finally, patient-specific determinants also play a significant role in the success of neoantigen vaccines. Genetic variation in patients, particularly the polymorphic MHC genes, results in variations in the binding affinities of neoantigens and, hence, in the ensuing T-cell responses [6]. Additionally, the general health and status of the immune system of the patient, which could be weakened by age, treatments, or other illnesses, will affect vaccine efficacy [41]. Such personalization needs to go a step further in designing vaccination strategies not only around the genetic makeup of the tumor but also around the immunological makeup of the patient. From a clinical point of view, several safety, efficacy, and regulatory approval issues emerge. Although neoantigens are tumor-specific and commonly safe, the chance of off-target effects may manifest when neoantigens cross-react with peptides originating from normal tissues [42]. This may give rise to autoimmune responses with immune cells targeting healthy tissues erroneously. Therefore, neoantigens require rigid screening to reduce the risk, which adds another dimension of intricacy to the creation of the vaccine [43]. As a consequence, further difficulties are faced in the design and implementation of neoantigen vaccines. The conventional design of clinical trials mostly utilizes huge cohorts receiving standard treatment, which may not be easily applicable for personalized therapies like neoantigen vaccines, where each patient receives a uniquely tailored product [44]. It requires new trial designs that can incorporate individual treatments and still allow for a strong inference in safety and efficacy. For this purpose, adaptive trial designs, which allow modifications of the trial protocol based on interim results, are under investigation [24]. Regulatory barrier could also be considered a significant concern. Regulatory agencies like. Whereas pathways for the approval of traditional drugs and biologics by both the FDA and EMA are established, the highly personalized nature of neoantigen vaccines does not easily fit into these frameworks [9]. These include a regulatory process that can accommodate the speed necessary for rapid turnaround from production to vaccination and, at the same time, responds to the highly individualized nature of the product. Developing new guidelines and frameworks that can cater to the peculiar characteristics of neoantigen vaccines is paramount for their successful translation into the clinic [23]. Navigating the regulatory landscape requires adapting to unique challenges presented by the individualized nature of neoantigen vaccines. However, there have been some notable successes that offer practical insights for researchers and developers. Provenge (Sipuleucel-T), developed by Dendreon Corporation, was the first personalized cancer vaccine approved by the FDA in 2010 for metastatic castration-resistant prostate cancer, marking a breakthrough in personalized immunotherapy [51]. It utilizes the patient’s dendritic cells exposed to a fusion protein to trigger an immune response against cancer cells. Provenge’s success, demonstrated by a significant median survival rate increase of 4.1 months in Phase III trials, paved the way for future personalized therapies, highlighting the importance of proving clinical benefits and addressing regulatory concerns [52]. Similarly, NeoVax, a neoantigen vaccine for melanoma, showed promising results in Phase I trials, gaining FDA approval for clinical trials and demonstrating the potential for personalized treatments [53]. Key lessons from these approvals include the importance of adaptive trial designs, establishing safety and efficacy, and scalable manufacturing practices. Engaging early with regulatory agencies, harmonizing clinical trial data, and leveraging real-world evidence are essential strategies for navigating regulatory pathways. Lastly, the cost and access associated with neoantigen vaccines pose a significant barrier. High costs are attributed to the development and production of personalized vaccines and logistic challenges in timely distribution to patients, which limit their access, especially in low-resource settings. [35]. Strategies to reduce costs, improve manufacturing efficiency, and streamline regulatory processes are essential to making these therapies more widely available [45].
This is a rapidly moving field due to technological advancements at an accelerated time of development for neoantigen-based cancer vaccines and also because of the increased insights into the biology of tumors. Such a new field holds great promise, with these vaccines now leaving the experimental setting and translating into clinical implementation. The present section discusses emerging vaccine platforms, strategies for overcoming resistance and relapse, and issues related to global access, equity, and scalability. The other critically important aspect is the emerging technologies that neoantigen vaccines are based on. Two of the most exciting platforms include mRNA-based vaccines and nanoparticle-based delivery systems. These vaccines have received a lot of attention recently and were successfully applied in COVID-19 vaccine development. Herein, the synthetic mRNA encoding the neoantigen is expressed directly into the cells of the patient, whereby this material is translated to protein. This protein is processed and presented to the immune system, thus eliciting a targeted immune response. mRNA platforms are flexible in this way, allowing fast design and production of vaccines that can be used for treatment matching according to patients’ tumors, making them highly compatible with personalized cancer therapy. Encouraging results have been published for early clinical trials using mRNA neoantigen vaccines in melanoma, showing strong T-cell responses and manageable safety profiles. The rapidity with which mRNA vaccines can be adapted to respond to an evolving antigenic landscape of the tumor will further position this platform as a potent tool in cancer immunotherapy [46]. Notably, gene-editing tools, particularly CRISPR-Cas9, hold exciting potential for enhancing neoantigen vaccines. The cellular genome of either tumor cells or immune cells can be edited by CRISPR to enhance its recognition and response to neoantigens. For example, CRISPR technology can be used to knock out genes that allow the bypass of the immune system by the tumor cells by lowering the expression of antigens. At the same time, CRISPR technology could also be used for editing T cells to modify their specificity for neoantigens, or synthetic receptors may be introduced that enhance the activation and persistence of T cells. Such gene-editing strategies can hold great potential in the betterment of neoantigen vaccines in eliciting a maximum number of tumor responses for immunotherapy-resistant tumors [47]. Nevertheless, even when considering such substantial prospects, resistance and relapse remain a formidable challenge to immunotherapy. One way to do this is by hitting multiple neoantigens in a single vaccine. Since neoplasms are highly heterogeneous, using this treatment for multiple neoantigens is a way to enhance complete tumor eradication, as it becomes more difficult for the tumor to evolve escape variants that do not express all antigens targeted. This approach can be particularly effective for relapse prevention, as it reduces the likelihood of residual tumor cells surviving and reinitiating recurrence [48]. Other strategies of neoantigen vaccination may include combinatorial approaches with other therapeutic modalities that will overcome resistance phenomena and further decrease the risk of relapse. Combinations of vaccines with immune checkpoint inhibitors will serve to sustain and magnify a vaccine-triggered immune response to prevent evasion of tumors from immune surveillance. Moreover, neoantigen vaccines combined with other immunotherapies, such as CAR-T cell therapy or TIL therapy, would be a multi-pronged attack on the tumor through different pathways and have a decreased chance of resistance to any one mechanism. Early clinical trials of such combinatory approaches are starting to show promise toward improving patient outcomes [24]. Incorporation of neoantigen vaccines into multi-modal treatment regimens that also involve surgery, radiation, and chemotherapy will increase their effectiveness. For example, a neoantigen vaccine given post-operation might eradicate the remaining cancer cells to prevent the emergence of metastasis. Likewise, combined with chemotherapy, the vaccine could synergize and give an overall anti-tumor effect. It is crucial that the timing and sequence of these treatments be aligned, and ongoing research is focused on the identification of optimal regimens for the maximal benefit of neoantigen vaccines [49]. As these neoantigen vaccinations have been increasingly integrated into the mainstream management of cancer, global access, equity, and scalability will need to be considered and ensured. However, personalized treatments, along with their infrastructure and cost requirements, as well as the need for prior regulatory approvals, face challenges that hinder their accessibility. This requires highly advanced infrastructure for the production of personalized neoantigen vaccines, including advanced sequencing technologies, bioinformatics platforms, and specialized manufacturing facilities capable of producing individualized vaccine formulations. Such infrastructure currently does not exist on a large scale in low- and middle-income countries. Meeting the challenges will require large investments in healthcare infrastructures, together with further technical development, to create new manufacturing processes that are both streamlined and more cost-effective to be feasible in a variety of settings [50]. Cost is one of the major hurdles for the general use of neoantigen vaccines. Their personalized nature, synthesis, and delivery are all expensive components, making access difficult, especially in settings with small health budgets. Most challenging, such as the creation of standardized, off- the-shelf components that could be easily tailored for each patient and automation in processes involved in vaccine manufacture, are cost-cutting strategies. Further, the public-private partnership would help in subsidizing some costs to ensure that these therapies are affordable to the patients who need them [35]. The regulatory environment for neoantigen vaccines is complicated, just like the field itself, and therefore differs vastly from place to place. Most of the national regulatory frameworks for approval of these therapies are under development, which is causing a delay in having access to innovative treatment. Harmonizing regulatory standards within regions or developing more streamlined pathways for the approval of personalized cancer vaccines will be essential to achieving these breakthroughs for patients worldwide. International regulatory bodies, like the FDA and EMA, could help partners elsewhere to speed up such reviews and approvals with assurances about safety and efficacy as soon as high standards are met [9]. Improving the scalability of neoantigen vaccines is also crucial for ensuring broader access. This includes developing manufacturing processes that can be scaled up to meet the needs of large populations and creating distribution networks that can promptly deliver these vaccines to patients. Advances in mRNA and nanoparticle technologies and innovations in vaccine storage and transport could play a critical role in enhancing scalability. Moreover, fostering local production capabilities in diverse regions could help reduce reliance on centralized manufacturing and improve the availability of neoantigen vaccines globally [23].
The development of neoantigen-based cancer vaccines represents a remarkable breakthrough in the field of immunotherapy and has the potential to bring new horizons for highly personalized cancer treatment. Genome sequencing and bioinformatics technologies for the exact identification of tumor-specific neoantigens have been integrated into vaccine design in the past decade. Clinical trials have demonstrated their potential to elicit strong and durable immune responses, particularly in cancers with high mutational burdens. However, many barriers lie in the way of wide adoption at the clinical level. Neoantigen identification, vaccine production, and personalized treatment can be technically difficult to facilitate, and at the same time, huge costs are associated with these therapies. The effectiveness of these vaccines is complicated by the biological hurdles linked to problems such as tumor immune escape. The regulatory frameworks are yet to evolve and be operational, given the peculiar nature of personalized therapy, and global access remains a critical issue. Continuous innovation in vaccine platforms, including mRNA and nanoparticle-based delivery systems, will be key to overcoming current limitations. Neoantigen vaccines and other immunotherapies, such as immune checkpoint inhibitors and adoptive cell therapies, give rise to potential strategies for increasing treatment effectiveness and reducing relapse rates. Throughout this process, the issues of global access and scalability must be discussed to bring into play neoantigen vaccines that will benefit every patient, regardless of their geography and economic constraints. Neoantigen vaccines hold promise for revolutionizing cancer therapy, moving the treatment of cancer from a very general approach to a highly individual strategy based on the molecular features of a given person’s tumor.
TAA – Tumor-Associated Antigen CEA – Carcinoembryonic Antigen PSA – Prostate-Specific Antigen MHC – Major Histocompatibility Complex NGS – Next-Generation Sequencing TMB – Tumor Mutation Burden FDA – Food and Drug Administration EMA – European Medicines Agency NSCLC – Non-Small Cell Lung Cancer GM-CSF – Granulocyte-Macrophage Colony-Stimulating Factor PAP – Prostatic Acid Phosphatase CTL – Cytotoxic T Lymphocytes APC – Antigen-Presenting Cells PD-L1 – Programmed Death-Ligand 1 CRISPR – Clustered Regularly Interspaced Short Palindromic Repeats CAR-T – Chimeric Antigen Receptor T-cells TME – Tumor Microenvironment
S.A.: Conceptualization, Design, Literature Review, Visualization, Manuscript Writing, Editing; M.S.M.: Literature review, Writing, Editing; M.F.S.: Literature Review, Writing, Editing.
This research did not receive any specific funding from public, commercial, or non-profit sectors.
We acknowledge that a pre-submission peer review was conducted using the ‘Peer Reviewer’ by Razia Aliani, a chatbot of OpenAI’s large language model ChatGPT. Additionally, ChatGPT was used before submission to improve the readability of this manuscript, with no further AI tools used thereafter.
Not Applicable.
The authors declare that they have no competing interests. No financial or personal relationships with other organizations or individuals that could have influenced the work reported in this paper exist.
[1] J. Ferlay, M. Colombet, I. Soerjomataram, D.M. Parkin, M. Piñeros, A. Znaor, et al., "Cancer statistics for the year 2020: An overview" Int. J. Cancer, vol. 149, pp. 778-789, 2021. [Crossref] [PubMed]
[2] Y. Zhang, Z. Zhang, "The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications" Cell. Mol. Immunol., vol. 17, pp. 807-821, 2020. [Crossref] [PubMed]
[3] I. Le, S. Dhandayuthapani, J. Chacon, A.M. Eiring, S.S. Gadad, "Harnessing the Immune System with Cancer Vaccines: From Prevention to Therapeutics" Vaccines, vol. 10, 2022. [Crossref] [PubMed]
[4] S. Wagner, C.S. Mullins, M. Linnebacher, "Colorectal cancer vaccines: Tumor-associated antigensvsneoantigens" World J. Gastroenterol., vol. 24, pp. 5418-5432, 2018. [Crossref]
[5] M. Kaczmarek, J. Poznańska, F. Fechner, N. Michalska, S. Paszkowska, A. Napierała, et al., "Cancer Vaccine Therapeutics: Limitations and Effectiveness—A Literature Review" Cells, vol. 12, 2023. [Crossref]
[6] N. Xie, G. Shen, W. Gao, Z. Huang, C. Huang, L. Fu, "Neoantigens: Promising targets for cancer therapy" Signal Transduct. Target. Ther., vol. 8, p. 9, 2023. [Crossref]
[7] C. Chakraborty, A. Majumder, M. Bhattacharya, S. Chatterjee, S.-S. Lee, "The landscape of neoantigens and its clinical applications: From immunobiology to cancer vaccines" Curr. Res. Biotechnol., vol. 7, 2024. [Crossref]
[8] F. Lang, B. Schrörs, M. Löwer, Ö. Türeci, U. Sahin, "Identification of neoantigens for individualized therapeutic cancer vaccines" Nat. Rev. Drug Discov., vol. 21, pp. 261-282, 2022. Correction in , , 156. doi:10.1038/s41573-023-00873-5 [Crossref]
[9] E. Blass, P.A. Ott, "Advances in the development of personalized neoantigen-based therapeutic cancer vaccines" Nat. Rev. Clin. Oncol., vol. 18, pp. 215-229, 2021. [Crossref]
[10] Z. Hu, D.E. Leet, R.L. Allesøe, G. Oliveira, S. Li, A.M. Luoma, et al., "Personal neoantigen vaccines induce persistent memory T cell responses and epitope spreading in patients with melanoma" Nat. Med., vol. 27, pp. 515-525, 2021. [Crossref]
[11] P. Pathak, S. Pajai, H. Kesharwani, "A Review on the Use of the HPV Vaccine in the Prevention of Cervical Cancer" Cureus, vol. 14, p. e28710, 2022. [Crossref] [PubMed]
[12] R. Kaczmarek, A.R. Pineros, P. E Patterson, T.B. Bertolini, G.Q. Perrin, A. Sherman, et al., "Factor VIII Trafficking to CD4+ T cells Shapes its Immunogenicity and Requires Several Types of Antigen Presenting Cells" Blood, vol. 142, pp. 290-305, 2023. [Crossref] [PubMed]
[13] N. Schaft, J. Dörrie, G. Schuler, B. Schuler-Thurner, H. Sallam, S. Klein, et al., "The future of affordable cancer immunotherapy" Front. Immunol., vol. 14, 2023. [Crossref] [PubMed]
[14] T. Fan, M. Zhang, J. Yang, Z. Zhu, W. Cao, C. Dong, "Therapeutic cancer vaccines: Advancements, challenges and prospects" Signal Transduct. Target. Ther., vol. 8, p. 450, 2023. [Crossref] [PubMed]
[15] Y. Li, M. Wang, X. Peng, Y. Yang, Q. Chen, J. Liu, et al., "mRNA vaccine in cancer therapy: Current advance and future outlook" Clin. Transl. Med., vol. 13, p. e1384, 2023. [Crossref]
[16] N. Ebrahimi, M. Akbari, M. Ghanaatian, P.R. Moghaddam, S. Adelian, M.B. Boroujeni, et al., "Development of neoantigens: From identification in cancer cells to application in cancer vaccines" Expert Rev. Vaccines, vol. 21, pp. 941-955, 2021. [Crossref]
[17] D.J. Verdon, M.R. Jenkins, "Identification and Targeting of Mutant Peptide Neoantigens in Cancer Immunotherapy" Cancers, vol. 13, 2021. [Crossref]
[18] G. Fotakis, Z. Trajanoski, D. Rieder, "Computational cancer neoantigen prediction: Current status and recent advances" Immuno-Oncol. Technol., vol. 12, 2021. [Crossref]
[19] Y. Cai, R. Chen, S. Gao, W. Li, Y. Liu, G. Su, et al., "Artificial intelligence applied in neoantigen identification facilitates personalized cancer immunotherapy" Front. Oncol., vol. 12, 2023. [Crossref]
[20] S. Kreiter, M. Vormehr, N. Van de Roemer, M. Diken, M. Löwer, J. Diekmann, et al., "Mutant MHC class II epitopes drive therapeutic immune responses to cancer" Nature, vol. 520, pp. 692-696, 2015. Correction in , , 370. doi:10.1038/nature14567 [Crossref]
[21] K. Kumari, A. Singh, A. Chaudhary, R.K. Singh, A. Shanker, V. Kumar, et al., "Neoantigen Identification and Dendritic Cell-Based Vaccines for Lung Cancer Immunotherapy" Vaccines, vol. 12, 2024. [Crossref] [PubMed]
[22] S.A. Rosenberg, N.P. Restifo, "Adoptive cell transfer as personalized immunotherapy for human cancer" Science, vol. 348, pp. 62-68, 2015. [Crossref] [PubMed]
[23] J. Liu, M. Fu, M. Wang, D. Wan, Y. Wei, X. Wei, "Cancer vaccines as promising immuno-therapeutics: Platforms and current progress" J. Hematol. Oncol., vol. 15, p. 28, 2022. [Crossref] [PubMed]
[24] P.A. Ott, Z. Hu, D.B. Keskin, S.A. Shukla, J. Sun, D.J. Bozym, et al., "An immunogenic personal neoantigen vaccine for patients with melanoma" Nature, vol. 547, pp. 217-221, 2017. Correction in , , 402. doi:10.1038/nature25145 [Crossref] [PubMed]
[25] P. Wang, Y. Chen, C. Wang, "Beyond Tumor Mutation Burden: Tumor Neoantigen Burden as a Biomarker for Immunotherapy and Other Types of Therapy" Front. Oncol., vol. 11, 2021. [Crossref]
[26] M.J.W. Sim, P.D. Sun, "T Cell Recognition of Tumor Neoantigens and Insights Into T Cell Immunotherapy" Front. Immunol., vol. 13, 2022. [Crossref]
[27] K. Li, H. Shi, B. Zhang, X. Ou, Q. Ma, Y. Chen, et al., "Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer" Signal Transduct. Target. Ther., vol. 6, p. 362, 2021. [Crossref]
[28] I. Malaina, L. Gonzalez-Melero, L. Martínez, A. Salvador, A. Sanchez-Diez, A. Asumendi, et al., "Computational and Experimental Evaluation of the Immune Response of Neoantigens for Personalized Vaccine Design" Int. J. Mol. Sci., vol. 24, 2023. [Crossref]
[29] A. Batista-Duharte, F. Hassouneh, P. Alvarez-Heredia, A. Pera, R. Solana, "Immune Checkpoint Inhibitors for Vaccine Improvements: Current Status and New Approaches" Pharmaceutics, vol. 14, 2022. [Crossref]
[30] R.R. Rapaka, A.S. Cross, M.A. McArthur, "Using Adjuvants to Drive T Cell Responses for Next-Generation Infectious Disease Vaccines" Vaccines, vol. 9, 2021. [Crossref]
[31] S. Turajlic, A. Sottoriva, T. Graham, C. Swanton, "Resolving genetic heterogeneity in cancer" Nat. Rev. Genet., vol. 20, pp. 404-416, 2019. Correction in , , 65. doi:10.1038/s41576-019-0188-1 [Crossref] [PubMed]
[32] M. Yarchoan, A. Hopkins, E.M. Jaffee, "Tumor Mutational Burden and Response Rate to PD-1 Inhibition" N. Engl. J. Med., vol. 377, pp. 2500-2501, 2017. [Crossref] [PubMed]
[33] M. Nielsen, M. Andreatta, B. Peters, S. Buus, "Immunoinformatics: Predicting Peptide–MHC Binding" Annu. Rev. Biomed. Data Sci., vol. 3, pp. 191-215, 2019. [Crossref] [PubMed]
[34] B.K. Bulik-Sullivan, P.R. Loh, H.K. Finucane, S. Ripke, J. Yang, N. Patterson, et al., "LD Score regression distinguishes confounding from polygenicity in genome-wide association studies" Nat. Genet., vol. 47, pp. 291-295, 2015. [Crossref] [PubMed]
[35] D.B. Keskin, A.J. Anandappa, J. Sun, I. Tirosh, N.D. Mathewson, S. Li, et al., "Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial" Nature, vol. 565, pp. 234-239, 2018. [Crossref]
[36] J.J. Havel, D. Chowell, T.A. Chan, "The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy" Nat. Rev. Cancer, vol. 19, pp. 133-150, 2019. [Crossref]
[37] N. McGranahan, C. Swanton, "Clonal Heterogeneity and Tumor Evolution: Past, Present, and the Future" Cell, vol. 168, pp. 613-628, 2017. [Crossref]
[38] Y. Kobayashi, A. Tata, A. Konkimalla, H. Katsura, R.F. Lee, J. Ou, et al., "Persistence of a regeneration-associated, transitional alveolar epithelial cell state in pulmonary fibrosis" Nat. Cell Biol., vol. 22, pp. 934-946, 2020. [Crossref]
[39] C. Huang, Y. Wang, X. Li, L. Ren, J. Zhao, Y. Hu, et al., "Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China" Lancet, vol. 395, pp. 497-506, 2020. Correction in , , 496. doi:10.1016/S0140-6736(20)30252-X [Crossref]
[40] S. Keshari, A.S. Shavkunov, Q. Miao, A. Saha, C.D. Williams, A.M. Highsmith, et al., "Neoantigen Cancer Vaccines and Different Immune Checkpoint Therapies Each Utilize Both Converging and Distinct Mechanisms that, in Combination Enable Synergistic Therapeutic Efficacy" bioRxiv, 2024. [Crossref]
[41] S.W. Duffy, J.P. Myles, R. Maroni, A. Mohammad, "Rapid review of evaluation of interventions to improve participation in cancer screening services" J. Med Screen., vol. 24, pp. 127-145, 2017. [Crossref] [PubMed]
[42] E.S. Borden, K.H. Buetow, M.A. Wilson, K.T. Hastings, "Cancer Neoantigens: Challenges and Future Directions for Prediction, Prioritization, and Validation" Front. Oncol., vol. 12, 2022. [Crossref] [PubMed]
[43] T.N. Schumacher, R.D. Schreiber, "Neoantigens in cancer immunotherapy" Science, vol. 348, pp. 69-74, 2015. [Crossref] [PubMed]
[44] C.J. Melief, T. van Hall, R. Arens, F. Ossendorp, S.H. van der Burg, "Therapeutic cancer vaccines" J. Clin. Investig., vol. 125, pp. 3401-3412, 2015. [Crossref] [PubMed]
[45] C.R. Reynolds, S. Tran, M. Jain, A. Narendran, "Neoantigen Cancer Vaccines: Generation, Optimization, and Therapeutic Targeting Strategies" Vaccines, vol. 10, 2022. [Crossref]
[46] U. Sahin, E. Derhovanessian, M. Miller, B.-P. Kloke, P. Simon, M. Löwer, et al., "Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer" Nature, vol. 547, pp. 222-226, 2017. [Crossref]
[47] T.L. Roth, C. Puig-Saus, R. Yu, E. Shifrut, J. Carnevale, P.J. Li, et al., "Reprogramming human T cell function and specificity with non-viral genome targeting" Nature, vol. 559, pp. 405-409, 2018. [Crossref]
[48] J.C. Castle, M. Uduman, S. Pabla, R.B. Stein, J.S. Buell, "Mutation-Derived Neoantigens for Cancer Immunotherapy" Front. Immunol., vol. 10, 2019. [Crossref]
[49] E.M. Van Allen, D. Miao, B. Schilling, S.A. Shukla, C. Blank, L. Zimmer, et al., "Genomic correlates of response to CTLA-4 blockade in metastatic melanoma" Science, vol. 350, pp. 207-211, 2015. Correction in , , aad8366. doi:10.1126/science.aad8366; Correction in , , aaf8264. doi:10.1126/science.aaf8264 [Crossref]
[50] R.E. Hollingsworth, K. Jansen, "Turning the corner on therapeutic cancer vaccines" Npj Vaccines, vol. 4, p. 7, 2019. [Crossref]
[51] M.A. Cheever, C.S. Higano, "PROVENGE (Sipuleucel-T) in Prostate Cancer: The First FDA-Approved Therapeutic Cancer Vaccine" Clin. Cancer Res., vol. 17, pp. 3520-3526, 2011. [Crossref] [PubMed]
[52] E. Anassi, U.A. Ndefo, "Sipuleucel-T (provenge) injection: The first immunotherapy agent (vaccine) for hormone-refractory prostate cancer" Pharm. Ther., vol. 36, pp. 197-202, 2011. [PubMed]
[53] J.V.L. Niemi, A.V. Sokolov, H.B. Schiöth, "Neoantigen Vaccines; Clinical Trials, Classes, Indications, Adjuvants and Combinatorial Treatments" Cancers, vol. 14, 2022. [Crossref] [PubMed]
[54] M. Weller, N. Butowski, D.D. Tran, L.D. Recht, M. Lim, H. Hirte, et al., "Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): A randomised, double-blind, international phase 3 trial" Lancet Oncol., vol. 18, pp. 1373-1385, 2017. [Crossref]
We use cookies to improve your experience on our site. By continuing to use our site, you accept our use of cookies. Learn more