
APA Style
Koki Yoshimoto, Ririka Okaichi, Kei Iida, Shiho Terada, Ken-ichiro Kamei. (2025). High-Purity Production of Endothelial Cells from Human Pluripotent Stem Cells. Cell Therapy & Engineering Connect, 1 (Article ID: 0006). https://doi.org/Registering DOIMLA Style
Koki Yoshimoto, Ririka Okaichi, Kei Iida, Shiho Terada, Ken-ichiro Kamei. "High-Purity Production of Endothelial Cells from Human Pluripotent Stem Cells". Cell Therapy & Engineering Connect, vol. 1, 2025, Article ID: 0006, https://doi.org/Registering DOI.Chicago Style
Koki Yoshimoto, Ririka Okaichi, Kei Iida, Shiho Terada, Ken-ichiro Kamei. 2025. "High-Purity Production of Endothelial Cells from Human Pluripotent Stem Cells." Cell Therapy & Engineering Connect 1 (2025): 0006. https://doi.org/Registering DOI.
 ACCESS
Research Article
ACCESS
Research Article
Volume 1, Article ID: 2025.0006

Koki Yoshimoto
yoshimoto.koki.5a@kyoto-u.ac.jp

Ririka Okaichi
2210330032b@kindai.ac.jp

Kei Iida
kiida@life.kindai.ac.jp

Shiho Terada
sterada@icems.kyoto-u.ac.jp

Ken-ichiro Kamei
kk4801@nyu.edu
1 Institute for Integrated Cell-Material Sciences, Kyoto University Institute for Advanced Study, Kyoto, 606-8501, Japan
2 Department of Life Science Faculty of Science and Engineering, Kindai University, 577-8502, Japan
3 Center for Cancer Immunotherapy and Immunobiology, Kyoto University Graduate School of Medicine, Kyoto 606-8501, Japan
4 Program of Biology, Division of Science, New York University Abu Dhabi, Abu Dhabi, P.O. Box 129188, United Arab Emirates
5 Program of Bioengineering, Division of Engineering, New York University Abu Dhabi, Abu Dhabi, P.O. Box 129188, United Arab Emirates
6 Department of Biomedical Engineering, Tandon School of Engineering, New York University, Brooklyn, NY 11201, USA
7 Department of Biology, Faculty of Arts & Science, New York University, New York, NY 10003, USA
8 Department of Pharmaceutics, Wuya College of Innovation, Shenyang Pharmaceutical University, Shenyang, 110016, China
9 Joint International Research Laboratory of Intelligent Drug Delivery Systems, Ministry of Education, Shenyang, 110016, China
* Author to whom correspondence should be addressed
Received: 01 Jun 2025 Accepted: 10 Oct 2025 Available Online: 10 Oct 2025
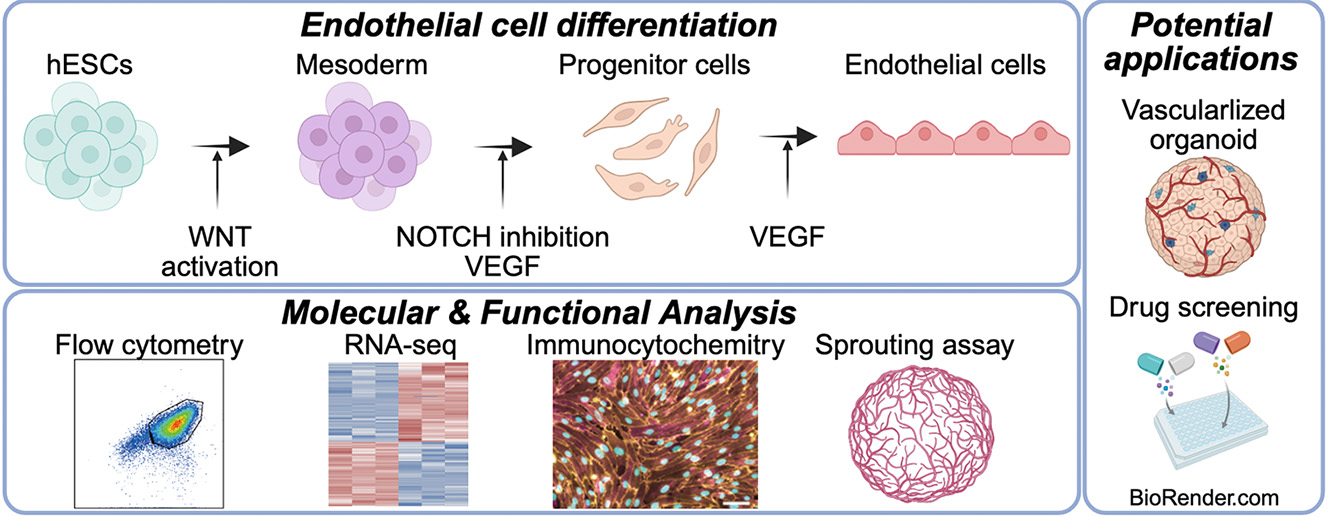
Endothelial cells (ECs) are essential for vascular network formation and tissue homeostasis, yet the fields of tissue engineering and vascularized organoid generation still relies heavily on human umbilical vein ECs (HUVECs), which are venous, allogeneic, and difficult to mature fully. Human pluripotent stem cells (hPSCs) offer an autologous, developmentally flexible alternative, but most differentiation protocols require fluorescence-activated cell sorting, limiting scalability. Here we present a streamlined method that produces highly pure ECs directly from human embryonic stem cells (hESCs) without cell sorting. Extending Wnt activation with CHIR99021 to three days maximizes mesoderm induction, and brief Notch blockade with DAPT during specification suppresses smooth-muscle commitment. The result is over 90 % CD31⁺ CD144⁺ ECs that display classic cobblestone morphology, robust DiI-acetylated LDL uptake, and capillary-like sprouting comparable to HUVECs. Bulk RNA barcoding and sequencing segregates the hESC-derived ECs from both HUVECs and undifferentiated hESCs and uncovers an artery-enriched transcriptome: NOTCH1, DLL4, and CXCR4 are up-regulated, whereas venous markers (EPHB4, NRP2) are reduced. Enrichment of Notch-responsive pathways further supports an arterial-like identity. Although several adult functional genes (e.g., vWF, NOS3) are expressed at lower levels than in HUVECs, the protocol delivers a scalable source of developmentally relevant ECs ideal for vascularizing organoids derived from the same hPSCs and for future applications in drug screening, disease modeling, and cell-based vascular therapies.
Disclaimer : This is not the final version of the article. Changes may occur when the manuscript is published in its final format.
We use cookies to improve your experience on our site. By continuing to use our site, you accept our use of cookies. Learn more