
APA Style
Nehad Jaser Ahmed. (2025). Descriptive Analysis of Reported Adverse Events Associated with Metoclopramide Using FDA Adverse Event Reporting System Databases. Clinical Pharmacy Connect, 1 (Article ID: 0002). https://doi.org/10.69709/CPC.2025.193994MLA Style
Nehad Jaser Ahmed. "Descriptive Analysis of Reported Adverse Events Associated with Metoclopramide Using FDA Adverse Event Reporting System Databases". Clinical Pharmacy Connect, vol. 1, 2025, Article ID: 0002, https://doi.org/10.69709/CPC.2025.193994.Chicago Style
Nehad Jaser Ahmed. 2025. "Descriptive Analysis of Reported Adverse Events Associated with Metoclopramide Using FDA Adverse Event Reporting System Databases." Clinical Pharmacy Connect 1 (2025): 0002. https://doi.org/10.69709/CPC.2025.193994.
 ACCESS
Research Article
ACCESS
Research Article
Volume 1, Article ID: 2025.0002

Nehad Jaser Ahmed
n.ahmed@psau.edu.sa
1 Department of Clinical Pharmacy, College of Pharmacy, Prince Sattam Bin Abdulaziz University, Alkharj 16278, Saudi Arabia
Received: 31 Jan 2025 Accepted: 03 Jun 2025 Available Online: 03 Jun 2025 Published: 23 Jun 2025
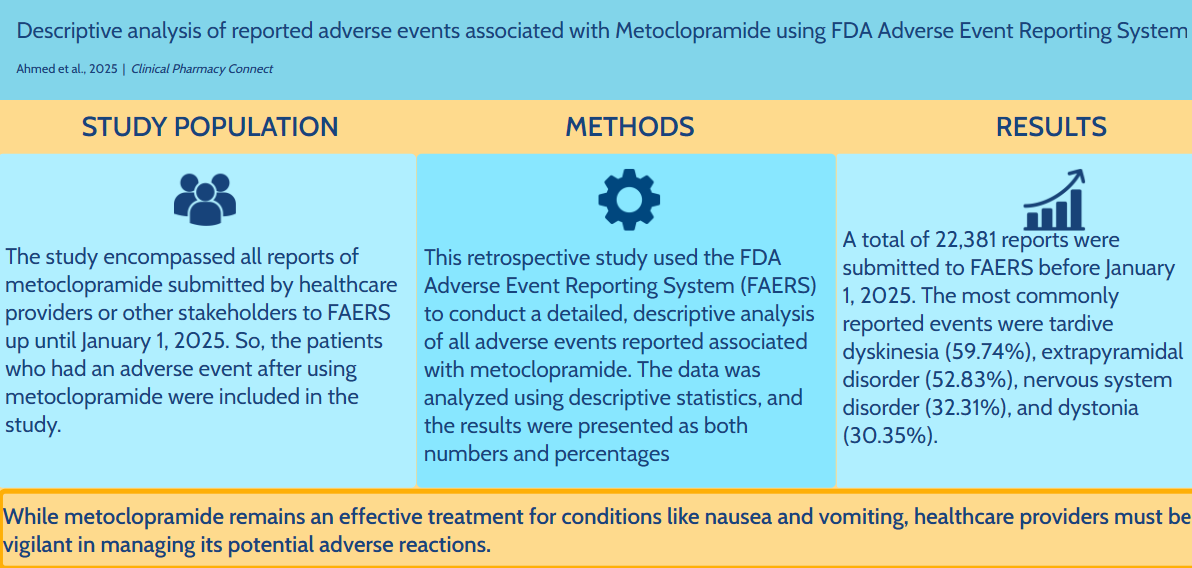
Metoclopramide has been approved by the Food and Drug Administration (FDA) for the treatment of nausea and vomiting in patients with gastroesophageal reflux disease (GERD) who are unresponsive to standard therapies, as well as for managing diabetic gastroparesis by enhancing gastric motility. This study aims to analyze and describe the adverse events associated with metoclopramide using the Food and Drug Administration Adverse Event Reporting System databases. The study encompassed all reports of metoclopramide submitted by healthcare providers or other stakeholders up until January 01, 2025. The data were analyzed using descriptive statistics, and the results were presented as both raw numbers and percentages. A total of 22,381 reports were submitted to FAERS before January 01, 2025. The majority of patients were female, accounting for 67.90%, and most of them were between the ages of 18 and 64. The most commonly reported events were tardive dyskinesia (59.74%), extrapyramidal disorder (52.83%), nervous system disorder (32.31%), and dystonia (30.35%). While metoclopramide remains an effective treatment for conditions like nausea and vomiting, healthcare providers must be vigilant in managing its potential adverse reactions.
Metoclopramide works by blocking central and peripheral dopamine D2 receptors in the medullary chemoreceptor trigger zone, specifically in the area postrema, which is typically activated by levodopa or apomorphine. This action reduces the sensitivity of visceral afferent nerves that relay signals from the gastrointestinal system to the vomiting center in the area postrema [1]. Besides its effects on dopamine receptors, metoclopramide also acts as an antagonist at 5HT3 (serotonin type 3) receptors and an agonist at 5HT4 receptors [2,3]. Additionally, it counteracts the antiperistaltic effects of apomorphine, which helps to mitigate its inhibition of gastric emptying. This leads to faster gastric emptying by increasing the amplitude and duration of esophageal contractions. Metoclopramide, therefore, increases the resting tone of the lower esophageal sphincter while relaxing the pyloric sphincter and duodenal bulb, thereby encouraging peristalsis in the duodenum and jejunum [4,5]. The Food and Drug Administration (FDA) has approved metoclopramide to treat diabetic gastroparesis by enhancing stomach motility, as well as to treat nausea and vomiting in patients with gastroesophageal reflux disease (GERD) who do not respond to standard therapy [6,7]. Further, when treating chemotherapy patients with nausea and vomiting, the FDA has approved the use of metoclopramide injections [8,9]. When nasogastric suction is not needed or advisable, off-label use of metoclopramide helps to stop vomiting and nausea after surgery. It is especially effective for this purpose because it does not increase gastric secretions [10]. Metoclopramide is also used off-label in the emergency room to treat acute migraines, acting as an effective adjuvant to reduce nausea, vomiting, and headache intensity. Although the FDA has not specifically approved it for acute migraine treatment, it is considered a valuable alternative to opioids, which carry a risk of use disorder [11,12]. Adverse events (AEs) are common in medical settings, affecting at least one in ten patients. An AE refers to a harmful outcome that occurs as a result of medical care, which could include procedures, surgeries, or medications. Any patient undergoing treatment might experience an adverse effect, which could range from medication side effects to physical injury, psychological harm, or even death [13]. A crucial component of the FDA’s systems is its post-marketing surveillance and risk assessment programs, which are designed to identify adverse events that may not have been evident during the drug approval process. It is impossible to anticipate every possible adverse effect because pre-approval studies usually only include a few hundred to a few thousand patients [14,15]. The FDA's post-marketing safety surveillance is supported by the FDA Adverse Event Reporting System (FAERS) Database. As mandated by law, it collects adverse event reports from consumers, healthcare providers, and manufacturers [16]. Metoclopramide is used extensively, but comprehensive research on its side effects, particularly extrapyramidal symptoms, is limited. Enhancing patient safety, directing clinical judgment, and influencing regulatory oversight all depend on an understanding of these adverse events. After a product is approved and put into use, the FDA Adverse Event Reporting System (FAERS) offers real-time data on drug safety as a type of post-marketing surveillance. This study reviews metoclopramide's side effects using FAERS, providing information about its safety profile beyond controlled clinical trials.
The researchers analyzed reports submitted to the FDA Adverse Event Reporting System (FAERS) for this retrospective study. As a key step in the FDA’s monitoring of drugs, FAERS combines vast data from healthcare professionals, patients, and manufacturers. The researchers examined these reports to identify risks and safety concerns related to metoclopramide observed in real-world clinical practice. The study included data for all adverse event reports associated with metoclopramide from the FDA Adverse Event Reporting System (FAERS) database that were submitted before January 01, 2025. The main sources of data were the FDA Adverse Event Reporting System (FAERS) public dashboard and quarterly data files. Reports where metoclopramide was the primary suspect drug were included to extract relevant cases. The search used the generic name “metoclopramide”, and did not limit by formulation. Adverse events were coded as Medical Dictionary for Regulatory Activities (MedDRA) Preferred Terms (PTs). The study considered various potential adverse events associated with metoclopramide. Particular attention was given to extrapyramidal and other neurological events. The selected reports for the present study contained the necessary information (drug name, suspected ADR, and demographic details of the patient). The analysis included all metoclopramide-related reports submitted by healthcare providers or other stakeholders before January 1, 2025. Any case that was submitted after this date was omitted from the analysis. Numerous factors linked to adverse events related to metoclopramide were investigated in the current study. The analysis examined the number of adverse event reports received and assessed their distribution over the years. In addition, details about the ages and genders of the patients, as well as the professional backgrounds of those filing the reports, were considered during the study. To highlight safety concerns and provide essential information to healthcare professionals, the research also assessed the most common related adverse events associated with metoclopramide. The data was examined using descriptive statistics, and the information was presented as percentages and numbers. The formula used to find the percentage for each group or category was (Part / Whole) × 100. This formula uses “Part” to show the reported numbers in a category and “Whole” to show the total number of reports. This approach offers a precise, numerical representation of the data distribution.
Before January 01, 2025, 22,381 reports were filed with FAERS. The distribution of these reports by year is shown in Table 1. The highest number of reports was submitted in 2011, accounting for 37.45% of the total, followed by 2012 with 22.70%. Roughly 15% of the reports were filed within the previous five years. The number of reports submitted during different years. Table 2 presents the age distribution of patients who experienced adverse events. The majority of patients were between the ages of 18 and 64, while 25.17% of the patients were aged between 65 and 85 years. The age of the patients. * In 16,008 reports, the age was not specified. Table 3 presents the gender distribution of patients who experienced adverse events. The majority of patients were female, making up 67.90%, while males accounted for 32.10%. The gender distribution of patients. * In 2545 reports, the gender was not specified. Table 4 presents the specialties of the individuals who submitted the reports. Only 29.21% of the reports were submitted by healthcare professionals, whereas the remaining 70.79% were reported by consumers. The specialties of the individuals submitting the reports. * In 619 reports, the specialties of the individuals submitting the reports were not specified. Table 5 displays the most frequently reported adverse events associated with metoclopramide. The most commonly reported events were tardive dyskinesia (59.74%), extrapyramidal disorder (52.83%), nervous system disorder (32.31%), and dystonia (30.35%). The most frequently reported adverse events associated with metoclopramide.
Category
Number of Cases
Percentage
2024
801
3.58%
2023
747
3.34%
2022
695
3.11%
2021
583
2.60%
2020
616
2.75%
2019
495
2.21%
2018
473
2.11%
2017
294
1.31%
2016
390
1.74%
2015
282
1.26%
2014
334
1.49%
2013
763
3.41%
2012
5080
22.70%
2011
8382
37.45%
2010
972
4.34%
Before 2010
1474
6.60%
Total
22,381
100.00%
Category *
Number of Cases
Percentage
0−1 Month
31
0.49%
2 Months−2 Years
91
1.43%
3−11 Years
122
1.91%
12−17 Years
220
3.45%
18−64 Years
4112
64.52%
65−85 Years
1604
25.17%
More than 85 Years
193
3.03%
Totals
6373
100.00%
Category *
Number of Cases
Percentage
Female
13,469
67.90%
Male
6367
32.10%
Totals
19,836
100.00%
Category *
Number of Cases
Percentage
Healthcare Professional
6356
29.21%
Consumer
15,406
70.79%
Totals
21,762
100.00%
Category
Number of Cases
Percentage
Tardive Dyskinesia
13,370
59.74%
Extrapyramidal Disorder
11,825
52.83%
Nervous System Disorder
7232
32.31%
Dystonia
6792
30.35%
Economic Problem
3768
16.84%
Pain
3653
16.32%
Movement Disorder
2893
12.93%
Loss of Personal Independence in Daily Activities
2891
12.92%
Anxiety
2703
12.08%
Incorrect Product Administration Duration
2564
11.46%
Dyskinesia
2533
11.32%
Deformity
2405
10.75%
Multiple Injuries
2200
9.83%
Decreased Quality of Life
1998
8.93%
Family Stress
1984
8.86%
Tremor
1781
7.96%
Emotional Distress
1755
7.84%
Fear
1680
7.51%
Akathisia
1606
7.18%
Anhedonia
1596
7.13%
Unevaluable Event
1098
4.91%
Emotional Disorder
1061
4.74%
Mental Disorder
955
4.27%
Nausea
824
3.68%
Drug Interaction
815
3.64%
The purpose of this study was to examine and characterize the side effects of metoclopramide. Most adverse event reports involving metoclopramide were filed between 2011 and 2012. The medication being prescribed less frequently or for shorter periods may have decreased the likelihood of adverse events, which could account for the recent decline in reports. To manage symptoms, Alkhowaiter et al. recommended the use of the lowest effective dose of metoclopramide for the shortest possible duration [17]. Furthermore, patients and healthcare professionals may now be more aware of the drug's possible adverse effects, which has improved management and decreased reporting. The present study showed that the majority of patients who reported metoclopramide side effects were either adults or the elderly. Adults, particularly those with chronic conditions or undergoing treatments that cause nausea (such as chemotherapy or post-surgery recovery), are more likely to use metoclopramide, which could explain the higher number of adverse event reports in this group. Lau Moon et al. stated that drug regulatory organizations in Canada and the European Union have changed the metoclopramide label, contraindicating its use in children under one year of age, cautioning against its use in children under five years, and advising against taking it for more than five days [18]. The present study found that the most commonly reported adverse events associated with metoclopramide were extrapyramidal symptoms, such as acute dystonia, parkinsonism, akathisia, and tardive dyskinesia, in addition to nervous system disorders. Previous studies have highlighted that, despite its effectiveness, metoclopramide may be linked to serious side effects, including extrapyramidal symptoms, hyperprolactinemia, and, in rare cases, neuroleptic malignant syndrome. Other common adverse events include headache, drowsiness, dizziness, diarrhea, and cardiac issues such as tachycardia, palpitations, and QT prolongation [19,20,21]. Lau Moon et al. reported that in children, the most frequently observed adverse effects are diarrhea and sedation [18]. Isola et al. and Donnet et al. found that extrapyramidal symptoms, such as torticollis, trismus, opisthotonus, akathisia, dystonia, oculogyric crisis, laryngospasm, hyperprolactinemia, tardive dyskinesia, parkinsonism, and neuroleptic malignant syndrome, are common adverse effects of metoclopramide use [22,23]. Gupta et al. noted that a rare but severe adverse event is neuroleptic malignant syndrome, which can present with hyperthermia, lead-pipe rigidity, leukocytosis, elevated creatine phosphokinase levels, altered consciousness, autonomic instability (e.g., diaphoresis, tachycardia, incontinence), pallor, irregular blood pressure or pulse, and cardiac arrhythmias [24]. Metoclopramide can also cause oculogyric crises and metoclopramide-induced hyperprolactinemia [25]. Additionally, metoclopramide may increase serum aldosterone levels, leading to fluid retention and potential volume overload, so it should be used cautiously in patients with heart failure or cirrhosis of the liver [26]. It has also been shown to cause a non-thrombocytopenic purpuric rash, which resolves after discontinuation of the drug [27]. Metoclopramide is extensively used and effective for treating nausea and vomiting. However, it is connected with various adverse consequences. According to Sridharan and Sivaramakrishnan, metoclopramide is especially effective in treating diabetic gastroparesis and postoperative nausea and vomiting. However, concerns regarding the danger of dyskinesias have spurred a rethinking of its application. Experts recommend that metoclopramide be started at the lowest possible dose and for the shortest term practicable. While it is beneficial in the short term, its central nervous system (CNS) side effects are a source of concern [28]. Isola et al. underlined that metoclopramide is routinely used in hospitals to treat gastroparesis, as well as postoperative nausea and vomiting, but healthcare practitioners should be aware of its possible risks. Thus, an interprofessional team approach is required. Before delivering the medicine, the nurse, pharmacist, and prescriber should obtain the patient's informed consent. Patients should be made aware of their risk of developing dyskinesias before starting treatment. The pharmacist should suggest a different course of treatment for the doctor to consider if the patient is more likely to experience adverse events [22]. 4.1. Strengths and Limitations The FDA Adverse Event Reporting System (FAERS), one of the biggest publicly accessible pharmacovigilance databases, is used in this study to provide significant insights into the adverse event profile of metoclopramide. This method's ability to evaluate a large volume of real-world data from various populations and clinical settings is one of its key advantages. It can assist in identifying possible safety signals that might not be apparent in carefully monitored clinical trials. The study does, however, have several limitations that are typical of spontaneous reporting systems such as FAERS. First, because not all adverse events, especially those that are mild or well-known, are recorded in the database, underreporting bias is a serious problem. Second, if duplicate reports are not appropriately handled during data analysis, they may artificially increase the perceived frequency of specific adverse events. Third, the perceived frequency of events may be distorted by reporting biases, such as a rise in submissions after safety alerts or media attention. Furthermore, an adverse event report does not prove beyond a reasonable doubt that metoclopramide caused the reaction, so FAERS data alone cannot establish causality. Finally, the computation of precise incidence rates is hindered by the lack of denominator data, such as the total number of patients exposed to the drug. Despite these constraints, the study’s findings enhance the existing safety literature on metoclopramide and highlight the importance of ongoing pharmacovigilance and clinical vigilance regarding its potential adverse effects. Future research should focus on complementing spontaneous reporting data with other sources, such as electronic health records, prescription databases, and prospective cohort studies, to gain a more complete understanding of the safety profile of metoclopramide. Studies aimed at quantifying the true incidence and risk factors for specific adverse events, especially extrapyramidal symptoms, are particularly needed. Additionally, further investigation into vulnerable populations (e.g., pediatric, elderly, or patients with comorbid neurological conditions) may help identify those at the highest risk. Finally, the development of predictive models using real-world data and machine learning techniques could enhance early signal detection and support more personalized medication safety strategies.
This descriptive analysis of adverse events connected with metoclopramide, which uses the FAERS database, demonstrates the significant risk of side effects associated with its use. The most commonly reported adverse effects were tardive dyskinesia, extrapyramidal diseases, nervous system disorders, and dystonia. These side effects highlight the significance of careful monitoring and dosing while administering metoclopramide, especially in high-risk patients. While metoclopramide remains an effective treatment for nausea and vomiting, healthcare practitioners must exercise caution while managing its potential side effects.
| 5HT3 | 5-Hydroxytryptamine 3 (Serotonin Type 3) |
| AEs | Adverse Events |
| CNS | Central Nervous System |
| FAERS | FDA Adverse Event Reporting System |
| FDA | Food and Drug Administration |
| GERD | Gastroesophageal Reflux Disease |
All data generated and analyzed are included in this research article.
Ethical approval and consent to participate do not apply to this study, as it is based on a synthesis of existing literature and does not involve human participants, animals, or original data collection.
No consent for publication is required, as the manuscript does not involve any individual personal data, images, videos, or other materials that would necessitate consent.
This study is supported via funding from Prince Sattam bin Abdulaziz University project number (PSAU/2024/R/1446).
Artificial intelligence (AI) tools were used solely to assist with grammatical refinement and language editing. All scientific analysis, data interpretation, and content development were conducted entirely by the author.
The author declares no conflicts of interest regarding this manuscript.
[1] Lee, A.; Kuo, B. Metoclopramide in the Treatment of Diabetic Gastroparesis. Expert Rev. Endocrinol. Metab. 2010, 5, 653–662. [CrossRef] [PubMed]
[2] Ge, S.; Mendley, S.R.; Gerhart, J.G.; Melloni, C.; Hornik, C.P.; Sullivan, J.E.; Atz, A.; Delmore, P.; Tremoulet, A.; Harper, B.; et al. Population Pharmacokinetics of Metoclopramide in Infants, Children, and Adolescents. Clin. Transl. Sci. 2020, 13, 1189–1198. [CrossRef] [PubMed]
[3] Sanger, G.J.; Andrews, P.L.R. A History of Drug Discovery for Treatment of Nausea and Vomiting and the Implications for Future Research. Front. Pharmacol. 2018, 9. [CrossRef] [PubMed]
[4] Ramsbottom, N.; Hunt, J.N. Studies of the Effect of Metoclopramide and Apomorphine on Gastric Emptying and Secretion in Man. Gut 1970, 11, 989–993. [CrossRef]
[5] Harada, T.; Hirosawa, T.; Morinaga, K.; Shimizu, T. Metoclopramide-Induced Serotonin Syndrome. Intern. Med. 2017, 56, 737–739. [CrossRef]
[6] Rettura, F.; Bronzini, F.; Campigotto, M.; Lambiase, C.; Pancetti, A.; Berti, G.; Marchi, S.; de Bortoli, N.; Zerbib, F.; Savarino, E.; et al. Refractory Gastroesophageal Reflux Disease: A Management Update. Front. Med. 2021, 8. [CrossRef]
[7] Shakhatreh, M.; Jehangir, A.; Malik, Z.; Parkman, H.P. Metoclopramide for the Treatment of Diabetic Gastroparesis. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 711–721. [CrossRef]
[8] Adel, N. Overview of Chemotherapy-Induced Nausea and Vomiting and Evidence-Based Therapies. Am. J. Manag. Care 2017, 23, S259–S265. [PubMed]
[9] Flank, J.; Robinson, P.D.; Holdsworth, M.; Phillips, R.; Portwine, C.; Gibson, P.; Maan, C.; Stefin, N.; Sung, L.; Dupuis, L.L. Guideline for the Treatment of Breakthrough and the Prevention of Refractory Chemotherapy-Induced Nausea and Vomiting in Children With Cancer. Pediatr. Blood Cancer 2016, 63, 1144–1151. [CrossRef]
[10] De Oliveira, G.S.; Castro-Alves, L.J.; Chang, R.; Yaghmour, E.; McCarthy, R.J. Systemic Metoclopramide to Prevent Postoperative Nausea and Vomiting: A Meta-Analysis Without Fujii’s Studies. Br. J. Anaesth. 2012, 109, 688–697. [CrossRef]
[11] Najjar, M.; Hall, T.; Estupinan, B. Metoclopramide for Acute Migraine Treatment in the Emergency Department: An Effective Alternative to Opioids. Cureus 2017, 9, e1181. [CrossRef] [PubMed]
[12] Eigenbrodt, A.K.; Ashina, H.; Khan, S.; Diener, H.C.; Mitsikostas, D.D.; Sinclair, A.J.; Pozo-Rosich, P.; Martelletti, P.; Ducros, A.; Lantéri-Minet, M.; et al. Diagnosis and Management of Migraine in Ten Steps. Nat. Rev. Neurol. 2021, 17, 501–514. [CrossRef]
[13] Skelly, C.L.; Cassagnol, M.; Munakomi, S. Adverse Events. Available online: https://www.ncbi.nlm.nih.gov/books/NBK558963/ (accessed on 31 January 2025).
[14] U.S. Food and Drug Administration Postmarketing Surveillance Programs. Available online: https://www.fda.gov/drugs/surveillance/postmarketing-surveillance-programs/ (accessed on 31 January 2025).
[15] Ahmed, N.J.; Fouda, M.I.; Fouda, D.I.; Foudah, A.I. The Adverse Effect Reporting for the Most Commonly Used Antibiotics. J. Pharm. Res. Int. 2020, 32, 22–28. [CrossRef]
[16] U.S. Food and Drug Administration FDA Adverse Event Reporting System (FAERS) Database. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-adverse-event-reporting-system-faers-database/ (accessed on 31 January 2025).
[17] Alkhowaiter, S.; Al Rasheed, M.M.; Alammar, N.; Alotaibi, A.; Altuwaijri, M.; Alshankiti, S.; Omair, M.A.; Alsahafi, M. Safety of prolonged use of metoclopramide and domperidone as treatment for chronic gastrointestinal dysmotility disorders in patients with systemic sclerosis. Saudi Pharm. J. 2024, 32, 102039. [CrossRef]
[18] Lau Moon Lin, M.; Robinson, P.D.; Flank, J.; Sung, L.; Dupuis, L.L. The Safety of Metoclopramide in Children: A Systematic Review and Meta-Analysis. Drug Saf. 2016, 39, 675–687. [CrossRef]
[19] Moos, D.D.; Hansen, D.J. Metoclopramide and Extrapyramidal Symptoms: A Case Report. J. Perianesth. Nurs. 2008, 23, 292–299. [CrossRef] [PubMed]
[20] Hale, T.W.; Kendall-Tackett, K.; Cong, Z. Domperidone Versus Metoclopramide. Clin. Lact. 2018, 9, 10–18. [CrossRef]
[21] Bashashati, M.; Sarosiek, I.; Siddiqui, T.; McCallum, R.W. Adverse Effects of Domperidone: Prolonged QuesT for Knowledge?. Dig. Dis. Sci. 2016, 61, 3384–3386. [CrossRef]
[22] Isola, S.; Hussain, A.; Dua, A.; Singh, K.; Adams, N. Metoclopramide. Available online: https://www.ncbi.nlm.nih.gov/books/NBK519517/ (accessed on 31 January 2025).
[23] Donnet, A.; Harle, J.R.; Dumont, J.C.; Alif Cherif, A. Neuroleptic Malignant Syndrome Induced by Metoclopramide. Biomed. Pharmacother. 1991, 45, 461–462. [CrossRef]
[24] Gupta, S.; Nihalani, N.D. Neuroleptic Malignant Syndrome: A Primary Care Perspective. Prim. Care Companion J. Clin. Psychiatry 2004, 6, 191–194. [CrossRef]
[25] Koban, Y.; Ekinci, M.; Cagatay, H.H.; Yazar, Z. Oculogyric Crisis in a Patient Taking Metoclopramide. Clin. Ophthalmol. 2014, 8, 567–569. [CrossRef] [PubMed]
[26] Nagahama, S.; Fujimaki, M.; Kawabe, H.; Nakamura, R.; Saito, I.; Saruta, T. Effect of Metoclopramide on the Secretion of Aldosterone and Other Adrenocortical Steroids. Clin. Endocrinol. 1983, 18, 287–293. [CrossRef] [PubMed]
[27] Upputuri, S.; Prasad, S. Metoclopramide-Induced Delayed Non-Thrombocytopenic Purpuric Rash. Clin. Drug Investig. 2006, 26, 745–747. [CrossRef] [PubMed]
[28] Sridharan, K.; Sivaramakrishnan, G. Interventions for Treating Nausea and Vomiting in Pregnancy: A Network Meta-Analysis and Trial Sequential Analysis of Randomized Clinical Trials. Expert Rev. Clin. Pharmacol. 2018, 11, 1143–1150. [CrossRef]
We use cookies to improve your experience on our site. By continuing to use our site, you accept our use of cookies. Learn more