
APA Style
Fakhra Alafeefi, Afsheen Raza. (2025). CRISPR-Cas9 is Revolutionizing AML Treatment as a Novel Therapy. Cancer Immunology Connect, 1 (Article ID: 0003). https://doi.org/10.69709/CIConnect.2025.103019MLA Style
Fakhra Alafeefi, Afsheen Raza. "CRISPR-Cas9 is Revolutionizing AML Treatment as a Novel Therapy". Cancer Immunology Connect, vol. 1, 2025, Article ID: 0003, https://doi.org/10.69709/CIConnect.2025.103019.Chicago Style
Fakhra Alafeefi, Afsheen Raza. 2025. "CRISPR-Cas9 is Revolutionizing AML Treatment as a Novel Therapy." Cancer Immunology Connect 1 (2025): 0003. https://doi.org/10.69709/CIConnect.2025.103019.
 ACCESS
Mini-Review
ACCESS
Mini-Review
Volume 1, Article ID: 2025.0003

Fakhra Alafeefi
Fakhraalafifi@gmail.com

Afsheen Raza
raza.afsheen@adu.ac.ae
1 Department of Biomedical Sciences, Abu Dhabi University, Abu Dhabi, United Arab Emirates
* Author to whom correspondence should be addressed
Received: 19 Dec 2024 Accepted: 18 Jan 2025 Available Online: 24 Jan 2025 Published: 11 Feb 2025
This review explores the progression of CRISPR-Cas9 genome editing technology in gene therapy for acute myeloid leukemia. Acute Myeloid Leukemia, a serious blood cancer, presents formidable treatment obstacles and a dismal prognosis. Effective treatments for acute myeloid leukemia rely on a clear understanding of its intricate genetic mechanisms, and CRISPR-Cas9 technology offers precision and adaptability for targeting the disease’s oncogenic drivers. The dual intron-targeting method of CRISPR-Cas9 is showcased in this review for its potential to improve acute myeloid leukemia treatment efficacy and decrease relapse risks. This approach emphasizes targeting oncogenic driver genes in acute myeloid leukemia through the dual intron-targeting CRISPR-Cas9 methodology, which identifies gene-specific targets, designs constructs to hit intronic regions, and explores viral and non-viral techniques for introducing CRISPR-Cas9 into leukemia cells. Preclinical model and primary acute myeloid leukemia cell evaluations indicate precise targeting and editing of oncogenic driver genes, significant reduction in cell viability and proliferation, minimal off-target effects, and improved treatment outcomes for acute myeloid leukemia patients. Finally, gene therapy, particularly CRISPR-Cas9, holds transformative potential in reshaping acute myeloid leukemia treatment. Despite challenges in optimizing delivery methods, enhancing target specificity, and minimizing off-target effects, CRISPR-Cas9 shows great promise in improving treatment efficacy and reducing relapse risk. This innovative approach offers hope for better survival rates and outcomes for acute myeloid leukemia patients, marking a significant advancement in the fight against this aggressive malignancy.
Acute Myeloid Leukemia (AML) is a formidable hematological cancer that significantly contributes to global morbidity and mortality. In 2020 alone, there were 474,519 new cases of leukemia worldwide, with 311,594 resulting in fatalities [1]. According to WHO [2], AML has a 5-year survival rate of 40%. The burden of AML has witnessed a marked increase over recent decades, with disability-adjusted life years attributed to this disease soaring by 56.14% from 1990 to 2017 [2]. At an age of 60 and beyond, the chances of surviving for five years or longer drop to under 20% [3]. In the US, approximately 20,800 new cases of AML are projected for 2024, along with around 11,220 related fatalities. Novel treatment approaches are essential for addressing the urgent need to improve outcomes for AML patients [1]. The effective management of AML, which includes chemotherapy and stem cell transplantation, continues to present challenges due to issues like treatment resistance and relapse. To combat the challenges facing AML treatment, it’s essential to discover new therapeutic options. In this introduction, we delve into the potential of gene therapy as an effective treatment for AML. Gene therapy has the potential to revolutionize AML management by directly targeting the underlying genetic aberrations driving leukemogenesis, thereby improving patient outcomes. This review explores the gene therapy mechanisms, its application in AML treatment, salient recent research findings, future possibilities, and existing obstacles. Gene therapy provides various ways to intervene in the genetic anomalies and signaling pathways causing leukemia. Some strategies target normal cellular function restoration, while others aim at inhibiting aberrant gene expression. Gene therapy can modulate the immune system to selectively target and eliminate leukemic cells. Although these approaches show promise, challenges like delivery efficiency and potential off-target effects still need to be addressed. Through a combination of these diverse techniques, gene therapy offers a novel and intricate solution for enhancing results in AML patients.
The literature review took a narrative approach to condense and evaluate research on AML, with a focus on CRISPR-Cas9 therapy as an innovative treatment option focusing on CRISPR-Cas9 therapy as an innovative option. Databases including PubMed, Google Scholar, BioMed Central, Frontiers in Oncology or Immunology, along with relevant websites, were searched using keywords such as “AML”, “Mutations”, and “CRISPR”. Preference was given to articles published between 2020 and 2024, focusing on peer-reviewed studies, clinical trials, and high-quality research. Titles, abstracts, and full texts were systematically reviewed to extract important findings related to clinical and therapeutic advancements. Moreover, ChatGPT (OpenAI, version GPT-4) was utilized for tasks such as shortening sentences, suggesting alternative words, and enhancing text fluency. Additionally, QuillBot’s AI assisted with rephrasing and grammar correction to enhance clarity. All changes made with these tools were carefully checked, corrected, and approved by the authors to ensure scientific accuracy and consistency with the manuscript’s objectives.
3.1. Mechanism of Action of AML AML arises from mutated marrow cells due to genetic alterations in their DNA. Leukemic blasts, also known as leukemic cells, fail to mature and differentiate into functional blood cells while multiplying excessively. Immature cells accumulate in the bone marrow and interfere with the production of normal blood cells Figure 1, leading to a decrease in healthy red cells, white cells, and platelets. Genetic mutations, whether spontaneous or induced by external factors including radiation, chemicals like benzene, or chemotherapy, are the primary cause of AML. AML arises from genetic mutations, resulting in leukemic blasts proliferation and normal haematopoiesis inhibition [4]. LSCs originate from altered self-renewing HSCs [5]. The intricate mutation and clonal evolution patterns revealed by next-generation sequencing have modified the traditional “two-hit hypothesis 3.2. AML Subtypes and Genetics AML subtypes are distinguished according to morphological, cytogenetic, and molecular features. Two primary classification systems are commonly used: FAB classification and WHO classification [4]. Based on their microscopic appearance and maturation stage, the FAB classification categorizes AML into eight distinct subtypes. AML subtypes include M0 (minimally differentiated), M1 (without maturation), M2 (with maturation), M3 (acute promyelocytic, features a chromosome 15-17 translocation), M4 (myelomonocytic, common with chromosome 16 translocation/inversion), M5 (monocytic), M6 (erythroleukemia), and M7 (megakaryocytic leukemia). The WHO classification improves the accuracy of FAB system by integrating cytogenetic and molecular data to better predict prognosis and guide treatment. This system encompasses multiple classes: AML marked by recurrent genetic alterations, as exemplified by t(8;21)(q22;q22) producing RUNX1-RUNX1T1 fusion gene, t(15;17)(q22;q12) resulting in the PML-RARA fusion gene characteristic of APL, and inv(16)(p13;q22) or t(16;16)(p13;q22) generating CBFB-MYH11 fusion gene; AML bearing myelodysplasia-related modifications post-therapy AML emerging after chemotherapy or radiation therapy; AML undefined (subdivided identically to the FAB hierarchy from M0 to M7); and AML carrying designated gene mutations (comprising NPM1, CEBPA, and FLT3-ITD). Genetic abnormalities can be categorized into risk groups, which affect the prognosis and treatment strategies for AML. Favourable outcomes are linked to abnormalities including t(8;21), t(15;17), and inv(16)/t(16;16), which create the RUNX1-RUNX1T1, PML-RARA, and CBFB-MYH11 fusion genes, respectively. Intermediate prognosis is linked with normal karyotype and MLLT3-MLL fusion gene caused by t(9;11). Patients with -5 or del(5q), -7, t(6;9)/DEK-NUP214, inv(3) or t(3;3)/RPN1-EVI1, or FLT3-ITD mutations have the least favourable prognosis [4]. 3.3. CRISPR/Cas9 and Gene Therapies in AML: Revolutionizing Genome Editing and Cancer Treatment A bacterial defensive system led to the development of the revolutionary genome-editing technology CRISPR/Cas9. It is an effective tool in genetics and biotechnology because it allows precise, focused alterations to DNA. The CRISPR/Cas9 system has a reputation for being able to integrate targeted modifications into genomes, which offers enormous potential for medical applications, agricultural practices, and basic biological research [9]. CRISPR stands for Clustered Regularly Interspaced Short Palindromic Repeats, and Cas9 stands for CRISPR-associated protein 9. This technology originates from the bacterial adaptive immune system, which uses the CRISPR/Cas9 mechanism to defend against viral infections. Bacteria incorporate DNA fragments from invading viruses into their own genome using CRISPR arrays, creating a genetic memory that allows them to recognize and defend against future infections by the same virus [9]. The CRISPR/Cas9 system identifies and cuts specific DNA sequences using RNA and protein components. The gRNA is crucial, directing the Cas9 protein to the target DNA sequence. The gRNA is composed of crRNA and tracrRNA sequences for target recognition and Cas9 binding, respectively. A sgRNA can now constitute both crRNA and tracrRNA in a single molecule. The gRNA directs Cas9 to the precise DNA sequence through base-pairing. The Cas9 enzyme can only bind and cleave a target sequence if it is neighboring a PAM sequence. Upon binding of gRNA-Cas9 complex to target DNA, Cas9 induces a DSB at the specified site as shown in Figure 1 [9]. The two nuclease domains of Cas9, RuvC2 and HNH3 [10], are in charge of cleaving DNA strands. The cell mends the double-strand break using its inherent DNA repair mechanisms. NHEJ and HDR are the primary repair mechanisms with NHEJ often resulting in gene function impairing indels. HDR allows for precise genomic modifications through the use of a repair template as shown in Figure 1 [9]. CRISPR/Cas9 is used across various industries for diverse applications. Genetic illnesses are treated by repairing their source mutations using gene therapies in their development. Genetically engineered crops with improved insect resistance and productivity can be developed through agriculture. Through CRISPR/Cas9 technology, scientists can create knockout models or introduce specific mutations in fundamental research for investigating gene function [9]. Its breakthrough capabilities come with challenges such as off-target consequences, which alter unwanted DNA sequences. Scientists are striving to enhance the system’s specificity and efficiency by developing new Cas9 variants, optimizing gRNA design, and employing complementary technologies such as base editors and prime editors. CRISPR/Cas9 represents a significant leap forward in genome editing due to its exceptional precision and versatility. Advances in research have the potential to revolutionize medicine, agriculture, and genetics, thereby determining the future of biotechnology [8]. 3.4. CRISPR-Cas9 in AML Treatment As previously mentioned, AML is a type of blood cancer known for its rapid and uncontrolled growth of immature myeloid cells, resulting from genetic mutations that disrupt key genes involved in blood cell production. Among these genetic irregularities, fusion genes resulting from CBF AML subtypes, such as inv(16)(p13.1q22)/t(16;16)(p13.1;q22) and t(8;21)(q22;q22.1) (AML t(8;21)), account for a large number of cases. These fusion genes, like the well-known RUNX1-RUNX1T1, play a key role in leukemia formation, as they interfere with normal cell activities and encourage the growth and spread of leukemia cells [11]. In AML treatment, common drugs like high-dose anthracycline and cytarabine are effective in causing remission but often fail to entirely eliminate leukemic cells, resulting in relapse in a significant number of patients. Furthermore, these therapies might cause considerable toxicity, especially in senior individuals. Therefore, there is a critical need for more targeted and less toxic treatment options, particularly those capable of effectively eliminating MRD to prevent relapse [11]. CRISPR-Cas9 technology represents a viable option in this regard. Researchers want to use the accuracy and adaptability of CRISPR-Cas9 to selectively target and disrupt oncogenic driver genes such as RUNX1-RUNX1T1 in AML cells [11]. The study by Neldeborg et al. [11] introduces a dual intron-targeting CRISPR-Cas9 methodology, which selectively disrupts fusion genes while maintaining the integrity of wild-type genes. This targeted approach could potentially prevent the growth and proliferation of AML cells by interfering with essential oncogenic pathways [11]. The findings show that CRISPR-Cas9 may effectively suppress the development and proliferation of AML t(8;21) cancer cells in vitro. Furthermore, the viability of this method is validated in primary AML cells obtained from patients, underscoring its potential for clinical use. By furnishing a focused and accurate means to disrupt oncogenic drivers in AML, CRISPR-Cas9 emerges as a promising therapeutic approach that could enhance treatment efficacy and mitigate the likelihood of relapse in AML patients. However, further studies are needed to assess its safety, scalability, and long-term effects before widespread implementation can be considered [11]. CRISPR/Cas technology, specifically CRISPR/Cas9, continues to improve its therapeutic potential and has great potential for gene therapy in AML. According to recent literature studies and clinical trial registry updates, many clinical trials are presently underway to investigate the use of CRISPR-Cas9 genome editing for AML treatment. Although the final results are still pending, several of these trials have shown promising encouraging initial outcomes. For instance, a Phase 1/2 ½ trial is currently recruiting participants for a therapeutic treatment involving autologous TCR T cells engineered to target WT1, which have been modified ex vivo using the CRISPR/Cas9 gene editing tool [12] (Trial No: NCT05066165). Another article by Chehelgerdi et al., published in 2024, presented outcomes from both preclinical and clinical trials, focusing on the application of CRISPR-based cancer treatment in AML cells. Preclinical trial data showed that the CRISPR approach of knocking out GATA2 induced differentiation and decreased tumor growth, while similar outcomes were observed following the knockout of ASXL1. However, both approaches are limited by insufficient evaluation of off-target effects [13]. In contrast, clinical trial results demonstrated that CAR-T cell therapy directed against CD33 in patients with R/R AML demonstrated positive outcomes. The process entails genetically modifying T cells to produce a CAR that targets CD33, after which they are administered to AML patients. While the therapy offers advantages like precise targeting and potential for a durable response, it also poses risks including cytokine release syndrome and neurotoxic effects [13,14]. Researchers are equipped with a multitude of genome editing options through the adaptable CRISPR/Cas toolbox, including Cas12, Cas13, and Cas14. However, research studies using CRISPR/Cas12-based treatments have shown encouraging results in treating illnesses such as sickle cell disease and beta-, which require blood transfusions, demonstrating successful integration with minimal adverse reactions. Furthermore, the discovery of other CRISPR/Cas cassettes, such Cas14, broadens the possibilities for more effective and adaptable gene editing approaches as shown in Figure 2. However, further exploration is required to fully exploit the therapeutic benefits of CRISPR/Cas9 and its variants in AML therapy. This entails overcoming challenges like optimizing delivery modalities, enhancing target specificity, and minimizing immunogenicity. The immune response to CRISPR-Cas9 poses a major obstacle to overcome in the context of therapeutic purposes. According to Kolanu [15], the introduction of the Cas9 protein into a living organism can initiate an immune response, causing the body to produce neutralizing antibodies and activate T-cells, which can limit the treatment’s effectiveness and safety. Recent studies have investigated numerous strategies to mitigate Cas9 immunogenicity. For instance, the researcher Charlesworth et al. demonstrated that engineering Cas9 protein through targeted modifications, such as introducing specific mutations into it, can significantly reduce its immunogenic properties and increase its therapeutic efficacy [15]. Additionally, researchers Chew et al. found that pre-existing immunity to Cas9 restrained the effectiveness of gene editing, highlighting the importance of considering a patient’s immunological history when creating CRISPR-Cas9-based treatments. Their study underscores the need for personalized approaches, including screening for existing immunity, prior to applying CRISPR-Cas9 in clinical settings. Another significant obstacle associated with CRISPR-Cas9 therapy is the risk of triggering an immune response due to the insertion of foreign genetic material. The immune system may identify ex-cell components as foreign entities, resulting in immune-related adverse effects and a decrease in the therapeutic effectiveness [15]. These findings indicate that overcoming immune response barriers is critical for successful CRISPR-Cas9 implementation in therapeutic settings. Future CRISPR-Cas9 research in AML should focus on understanding the potential of different delivery systems and their efficacy in transducing AML cells. The success of CRISPR-Cas9-mediated disruption of fusion genes as a therapy for AML relies on the efficient delivery of its components to leukemic cells in vivo.. An unexpected finding of Cas9 cleavage in the THP1 cell line, which resulted in the creation of a t(8;21) translocation, highlights the need for additional research into the safety and specificity of CRISPR-Cas9 applications. Although this discovery did not lead to growth suppression or major negative effects in vitro tests, the possibility of creating gene fusions in unintended cells requires careful scrutiny, especially in preclinical models. Comprehensive evaluation of potential side effects, particularly the induction of translocations, is important for understanding their impact on healthy cells and for developing strategies to minimize these risks in future applications as shown in Figure 2. Despite demonstrating efficacy in inhibiting proliferation and reducing tumor volume in AML cells with CRISPR-Cas9 dual intron-targeting in AML t(8;21) [11], addressing these challenges is crucial for bringing this technology into clinical use. In order to ensure that CRISPR-Cas9 is safe and effective when implemented in AML t(8;21) patients, future studies should optimize delivery strategies, assess efficacy in combination with existing treatments, and conduct comprehensive off-target effects analyses as seen in Figure 2 [9,11].![Figure 1: Mechanism of action of CRISPR/Cas9 gene therapy in AML, highlighting targeted DNA cleavage and repair pathways for precise genome editing [7,8].](/uploads/source/articles/cancer-immunology-connect/2025/volume1/2025002025.0003/image001.png)
4. Future Perspectives and Challenges
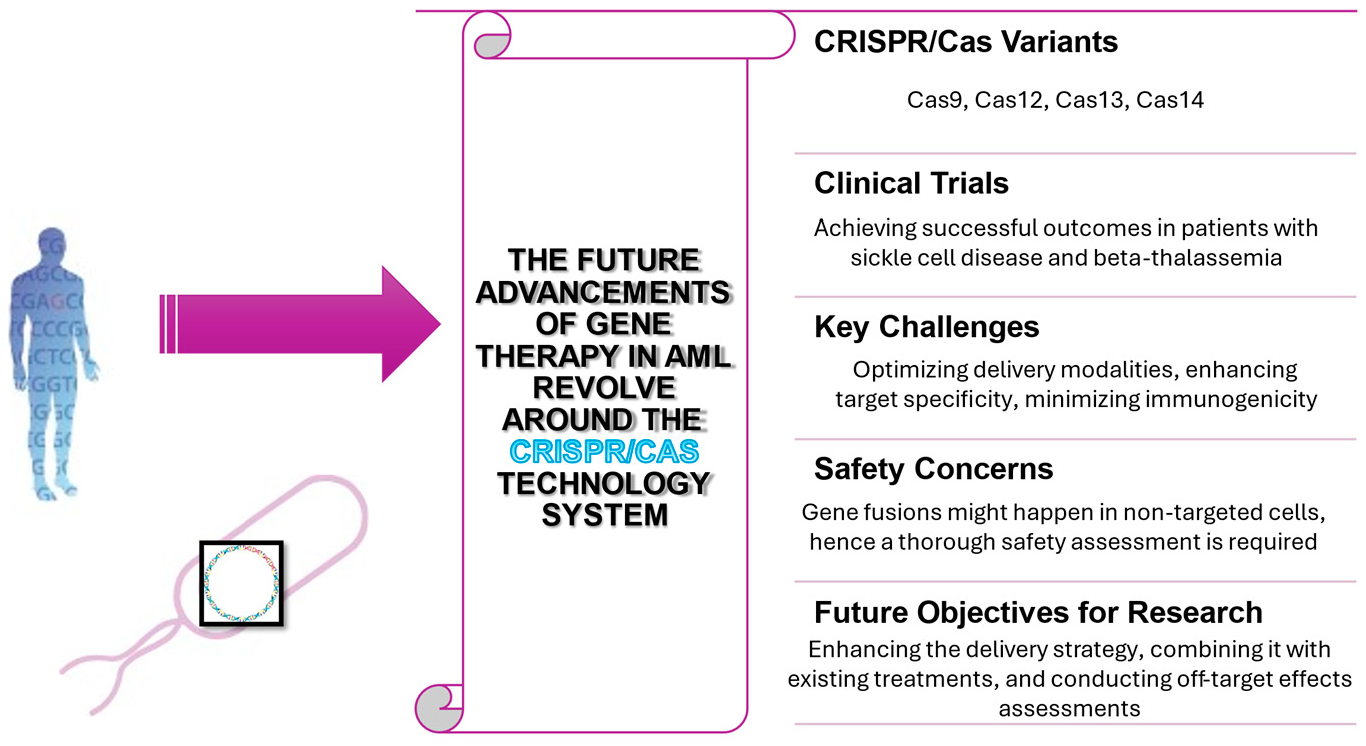
Finally, this paper emphasizes how CRISPR-Cas9 technology can revolutionize the way that AML is treated. Despite the challenges posed by high relapse rates and treatment resistance, CRISPR-Cas9 represents a potential step toward more effective personalized therapies. By precisely targeting genetic defects and disrupting cancer-causing processes, this technology has shown significant potential in preclinical models, particularly through approaches like dual intron-targeting to tackle fusion genes essential for AML pathogenesis. As previously stated, AML is caused by complex genetic changes and clonal formation. Preclinical models indicate that CRISPR-Cas9’s ability to generate targeted double-strand breaks and subsequent genetic modifications can precisely target AML’s underlying mechanisms, reducing leukemic cell proliferation and decreasing tumor volume. This technology can disrupt fusion genes like RUNX1-RUNX1T1, essential for AML pathogenesis, through a dual intron-targeting approach. Transitioning from lab to patient care poses considerable challenges. Ensuring delivery efficiency, specificity to targets, and minimal off-target effects remains essential. To ensure safety and specificity, extensive preclinical research is necessary due to the likelihood of unintended gene fusions with CRISPR-Cas9 applications. Effectively adopting CRISPR-based medicines in clinical practice relies on overcoming the identified challenges. Further investigation is required to enhance gene-editing medication delivery systems, assess the long-term safety and efficacy, and combine CRISPR-Cas9 with present therapeutic approaches. The discovery of new CRISPR/Cas systems, such as Cas12 and Cas14, and advances in this technology offer opportunities for advanced, efficient therapeutic applications. Despite the obstacles to practical application, CRISPR-Cas9 has the potential to transform the treatment of AML. By addressing the genetic basis of leukemogenesis, gene therapy holds the potential to transform the treatment of AML, enhancing survival rates and improving the quality of life for patients. As research advances, gene therapy incorporated into AML treatment could become pivotal to combating this aggressive hematologic malignancy.
APL
Acute Promyelocytic Leukemia
AML
Acute Myeloid Leukemia
AML t(8;21)
Acute Myeloid Leukemia with t(8;21) translocation
CBF
Core-Binding Factor
CRISPR
Clustered Regularly Interspaced Short Palindromic Repeats
Cas9
CRISPR-associated protein 9
CRISPR RNA
crRNA
DSB
Double-Strand Break
DNA
Deoxyribonucleic Acid
FAB
French-American-British
FLT3-ITD
FMS-like Tyrosine Kinase 3 - Internal Tandem Duplication
gRNA
guide RNA
HDR
Homology-Directed Repair
HSCs
Hematopoietic Stem Cells
Indels
Insertions or Deletions
LLS
Leukemia & Lymphoma Society
LSCs
Leukemia Stem Cells
MRD
Minimal Residual Disease
MSCs
Mesenchymal Stem Cells
NHEJ
Non-Homologous End Joining
PAM
Protospacer Adjacent Motif
RNA
Ribonucleic Acid
R/R
Relapsed/Refractory
sgRNA
single guide RNA
tracrRNA
Trans-Activating CRISPR RNA
WHO
World Health Organization
A.R. provided valuable insights into manuscript preparation and organization. F.A. conducted the scientific literature search, designed the review structure, and wrote the document. A.R. contributed to manuscript writing, assisted with design, and reviewed the final version. All authors have read and approved the final manuscript.
The authors declare no conflicts of interest.
No external funding was received for this article.
We would like to acknowledge the American Society of Hematology (ASH) Image Bank for providing an extensive collection of hematologic images, which significantly enhanced this review and improved the quality of this work. Finally, the authors acknowledge the use of ChatGPT (OpenAI, version GPT-4) for text editing when preparing the manuscript, and QuillBot’s AI for paraphrasing and grammar checking.
[1] "American Cancer Society" Available online: https://www.cancer.org/cancer/types/acute-myeloid-leukemia/about/key-statistics.html. (accessed on 18 February 2024)
[2] L. Ma, Q. Wang, X. Li, Y. Shang, N. Zhang, J. Wu, et al., "Development of a risk assessment model for cardiac injury in patients newly diagnosed with acute myeloid leukemia based on a multicenter, real-world analysis in China" BMC Cancer, vol. 24, 2024. [Crossref] [PubMed]
[3] "How Long Will I Live with Acute Myeloid Leukemia? - HealthTree for Acute Myeloid Leukemia" Available online: https://healthtree.org/aml/community/how-long-will-i-live-with-acute-myeloid-leukemia. (accessed on 18 February 2024)
[4] L.J. DegennaroL.J. Degennaro, "Acute Myeloid Leukemia" Available online: https://www.lls.org/sites/default/files/file_assets/aml.pdf. (accessed on 20 February 2024)
[5] F. Wachter, Y. Pikman, "Pathophysiology of Acute Myeloid Leukemia (AML)" Acta Haematol., vol. 147, pp. 229-246, 2024. [Crossref]
[6] S. SubramanianS. Subramanian, "News-Medical.Net" Available online: https://www.news-medical.net/life-sciences/What-are-Stromal-Cells.aspx#:~:text=Stromal%20cells%20%E2%80%93%20also%20known%20as,%2C%20ectoderm%2C%20and%20endoderm. (accessed on 15 March 2024)
[7] P. MaslakP. Maslak, "American Society of Hematology (ASH) │ Image Bank" Available online: https://imagebank.hematology.org/collection/2212. (accessed on 21 December 2024)
[8] J. Jazayerli, G. VenkataramanJ. Jazayerli, G. Venkataraman, "American Society of Hematology (ASH) │ Image Bank" Available online: https://imagebank.hematology.org/collection/64070. (accessed on 21 December 2024)
[9] M. Tyumentseva, A. Tyumentsev, V. Akimkin, "CRISPR/Cas9 Landscape: Current State and Future Perspectives" Int. J. Mol. Sci., vol. 24, 2023. [Crossref] [PubMed]
[10] C. Xue, E.C. Greene, "DNA repair pathway choices in CRISPR-Cas9 mediated genome editing" Trends Genet., vol. 37, pp. 639-656, 2021. [Crossref]
[11] S. Neldeborg, J.F. Soerensen, C.T. Møller, M. Bill, Z. Gao, R.O. Bak, et al., "Dual intron-targeted CRISPR-Cas9-mediated disruption of the AML RUNX1-RUNX1T1 fusion gene effectively inhibits proliferation and decreases tumor volume in vitro and in vivo" Leukemia, vol. 37, pp. 1792-1801, 2023. [Crossref]
[12] S. Chira, A. Nutu, E. Isacescu, C. Bica, L. Pop, C. Ciocan, et al., "Genome Editing Approaches with CRISPR/Cas9 for Cancer Treatment: Critical Appraisal of Preclinical and Clinical Utility, Challenges, and Future Research" Cells, vol. 11, 2022. [Crossref]
[13] M. Chehelgerdi, M. Chehelgerdi, M. Khorramian-Ghahfarokhi, M. Shafieizadeh, E. Mahmoudi, F. Eskandari, et al., "Comprehensive review of CRISPR-based gene editing: mechanisms, challenges, and applications in cancer therapy" Mol. Cancer, vol. 23, p. 9, 2024. [Crossref] [PubMed]
[14] H. Acharya, B. Walter, "Chimeric Antigen Receptor (CAR)-Modified Immune Effector Cell Therapy for Acute Myeloid Leukemia (AML)" Cancers, vol. 12, 2020. [Crossref] [PubMed]
[15] N.D. Kolanu, "CRISPR–Cas9 Gene Editing: Curing Genetic Diseases by Inherited Epigenetic Modifications" Global Medical Genetics, vol. 11, pp. 113-122, 2024. [Crossref] [PubMed]
We use cookies to improve your experience on our site. By continuing to use our site, you accept our use of cookies. Learn more