
APA Style
Fathima Hasnain Nadeem Hussain, Khadija Shafique, Afsheen Raza. (2025). Soluble Biomarkers as Predictors of Response to Immunotherapy in Non-Small Cell Lung Cancer (NSCLC)-Insights from the Tumor Microenvironment Perspective. Cancer Immunology Connect, 1 (Article ID: 0004). https://doi.org/10.69709/CIConnect.2025.003399MLA Style
Fathima Hasnain Nadeem Hussain, Khadija Shafique, Afsheen Raza. "Soluble Biomarkers as Predictors of Response to Immunotherapy in Non-Small Cell Lung Cancer (NSCLC)-Insights from the Tumor Microenvironment Perspective". Cancer Immunology Connect, vol. 1, 2025, Article ID: 0004, https://doi.org/10.69709/CIConnect.2025.003399.Chicago Style
Fathima Hasnain Nadeem Hussain, Khadija Shafique, Afsheen Raza. 2025. "Soluble Biomarkers as Predictors of Response to Immunotherapy in Non-Small Cell Lung Cancer (NSCLC)-Insights from the Tumor Microenvironment Perspective." Cancer Immunology Connect 1 (2025): 0004. https://doi.org/10.69709/CIConnect.2025.003399.
 ACCESS
Review Article
ACCESS
Review Article
Volume 1, Article ID: 2025.0004

Fathima Hasnain Nadeem Hussain
fathima279h@gmail.com

Khadija Shafique
700045914@uaeu.ac.ae

Afsheen Raza
raza.afsheen@adu.ac.ae
1 Department of Biomedical Sciences, College of Health Sciences, Abu Dhabi University, Abu Dhabi 59911, United Arab Emirates
2 Department of Genetics and Genomics, College of Medicine and Health Sciences, United Arab Emirates University, Al-Ain 15551, United Arab Emirates
* Author to whom correspondence should be addressed
Received: 06 Dec 2024 Accepted: 03 Feb 2025 Available Online: 10 Feb 2025 Published: 19 Mar 2025
Lung cancer is the major cause of mortality worldwide with non-small cell lung cancer (NSCLC) contributing to 85% of the cases. FDA-approved immune checkpoint inhibitors (ICI), anti-PD-1, and anti-PD-L1 are widely used as a standard treatment. However, the response rate is limited and is observed in only 20–40% of the patients. Therefore, predictive and prognostic biomarkers are needed to help differentiate responders from non-responders, enabling a better understanding of disease dynamics and facilitating more effective management through the optimal use of these valuable drugs. In this context, tissue-based biomarkers have several limitations such as invasive sampling, tumor heterogeneity and difficulty in longitudinal monitoring. Therefore, non-invasive blood-based biomarkers could serve as impactful tools as predictive and prognostic biomarkers in ICI-treated NSCLC patients. In lieu of this, this review article aims to provide updated insights on the utility of soluble molecules such as soluble immune checkpoints, exosomes and cytokines as prognostic and predictive biomarkers of response in ICI-treated NSCLC patients.
Lung cancer is the leading cause of cancer-related deaths (18% of total cancer deaths) worldwide. It has high morbidity with 70% of patients diagnosed with advanced disease, while only 15% remain alive five years after diagnosis [1]. Lung cancer is classified into Small Cell Lung cancer (SCLC) and Non–Small Cell Lung cancer (NSCLC). Of these two histological types, NSCLC is the most common type with a diagnosis rate of approximately 85% [2]. Smoking is the most significant risk factor for NSCLC, however in non-smokers genetic predispositions along with environmental factors can also have an impact on the individual [3]. Various subtypes of NSCLC differ in genetic alterations, treatment and histological features [4,5]. Therefore, NSCLC treatment varies accordinglyand may include a combination of chemotherapy and high-dosage radiotherapy. Adverse events associated with such treatments pose risks of adverse effects compromising quality of life [2]. In addition, drug resistance and mutational accumulation with immunosuppressive factors at play enable metastasis and progression leading to poor outcomes. Patients with positive mutational profiles for Epidermal growth factor receptor (EGFR), v-Raf murine sarcoma viral oncogene homolog B (BRAF) inhibitors targeting BRAF can be treated with targeted therapies [6]. However, patients with no genetic mutations are treated primarily with FDA approved immune checkpoint inhibitors (ICI) [7]. Immune checkpoint inhibitors (ICI) main mode of action is to block and target mediators of the immune checkpoint pathway; Programmed Death Ligand-1 (PDL-1) and Programmed Cell Death protein-1 (PD-1). Briefly, the immune checkpoint pathway has a role in preventing T cell activation through down-regulation of the immune system to promote self-tolerance/prevent autoimmunity. Therefore, blocking this pathway via immune checkpoint inhibitors helps to unleash the immune response and augment robust T cell response against the tumor [8,9]. Though immune checkpoint inhibitors have proven to be a paradigm shift for better overall survival in NSCLC patients, response rates to ICI have been less than optimal with only 20–40% of the patients responding to this treatment [10]. This lack of response to ICIs has been associated with several intrinsic and extrinsic factors, including tumor antigen absence leading to lack of tumor recognition by T cells, alterations in the antigen presenting machinery, and most importantly, the manipulated cellular components of the tumor microenvironment. These components include regulatory T cells (T regs), myeloid-derived suppressor cells (MDSCs), M2 macrophages, inhibitory immune checkpoints, cytokines, and chemokines that modulate the immune system to facilitate tumorigenesis [10]. Recently, several studies have documented the role of soluble forms of immune checkpoints, T cell and NK cell receptors and ligands, cytokines, and exosomes as contributors to resistance to ICI treatment [11,12,13,14]. It is postulated that these soluble mediators, secreted within the tumor microenvironment, hinder treatment dynamics by exerting their inhibitory effects either by binding to the treatment active site or activating immune suppressive molecules to facilitate tumor immune escape [15] (Table 1, Figure 1). It is postulated that these soluble mediators can serve as valuable biomarkers in stratifying responding and non-responding patients, thus, allowing a better understanding of disease and treatment dynamics. Furthermore, these soluble markers are advantageous with respect to their utility in longitudinal treatment monitoring with easily accessible biological samples, such as serum or plasma [11,12,13]. Therefore, in this review, the aim is to provide updated insights into the role of soluble PD-1, PD-L1, PD-L2, CTLA4, cytokines and exosomes as biomarkers of response to immunotherapy in NSCLC. Mechanism of action of soluble PD-1, PD-L1 and PD-L2, sCTLA-4, sLAG-3 and exosomes.
Soluble Marker
Effect on Immune Microenvironment
sPD-1
Immune enhancement and inhibitory effect:
Early activation of CD8+lymphocytes
Boost the lytic activity of macrophages.
Upregulation of TNF-α, IFN-γ, INOS
Reduce OX40, IL-10 expression.
Blocks PD-L1expression on tumor cells
Reverse signaling on dendritic cells (DC)
Reduction in DC maturation
Inhibition of IL-2 production/T cell activation
sPD-L1
Immune Inhibitory effects:
T cell apoptosis
Immune suppression
Negative regulation of T-cell function
Inhibition of lymphocyte function
Inhibition of T-cell proliferation/IFN-gamma production
Reduction in Th1 cytokines
Increase in Th2 cytokines expression
sPD-L2
Immune inhibitory and enhancement effects:
Decreased cytokine production by CD4+T cells
Inhibition of T cell receptor (TCR)-mediated proliferation
Activation of T cells via IL-12 production
sCTLA-4
Potent immune modulator:
Blocks CD28-B7 interaction.
Suppresses antigen-driven proliferation
Reducing cytokine release.
sLAG-3
Dual potential in modulating immune responses:
Promotes DC maturation by binding to MHC-II
Decreases monocyte development
Reduced antigen presentation abilities
Inhibits T cell proliferation
Exosomal PDL1
Immune Inhibitory effects:
Inhibits CD8+ activity and T cell activation
Inhibits monocyte differentiation
Converts myeloid precursor cells to myeloid suppressor cells
Suppresses the function of NK cells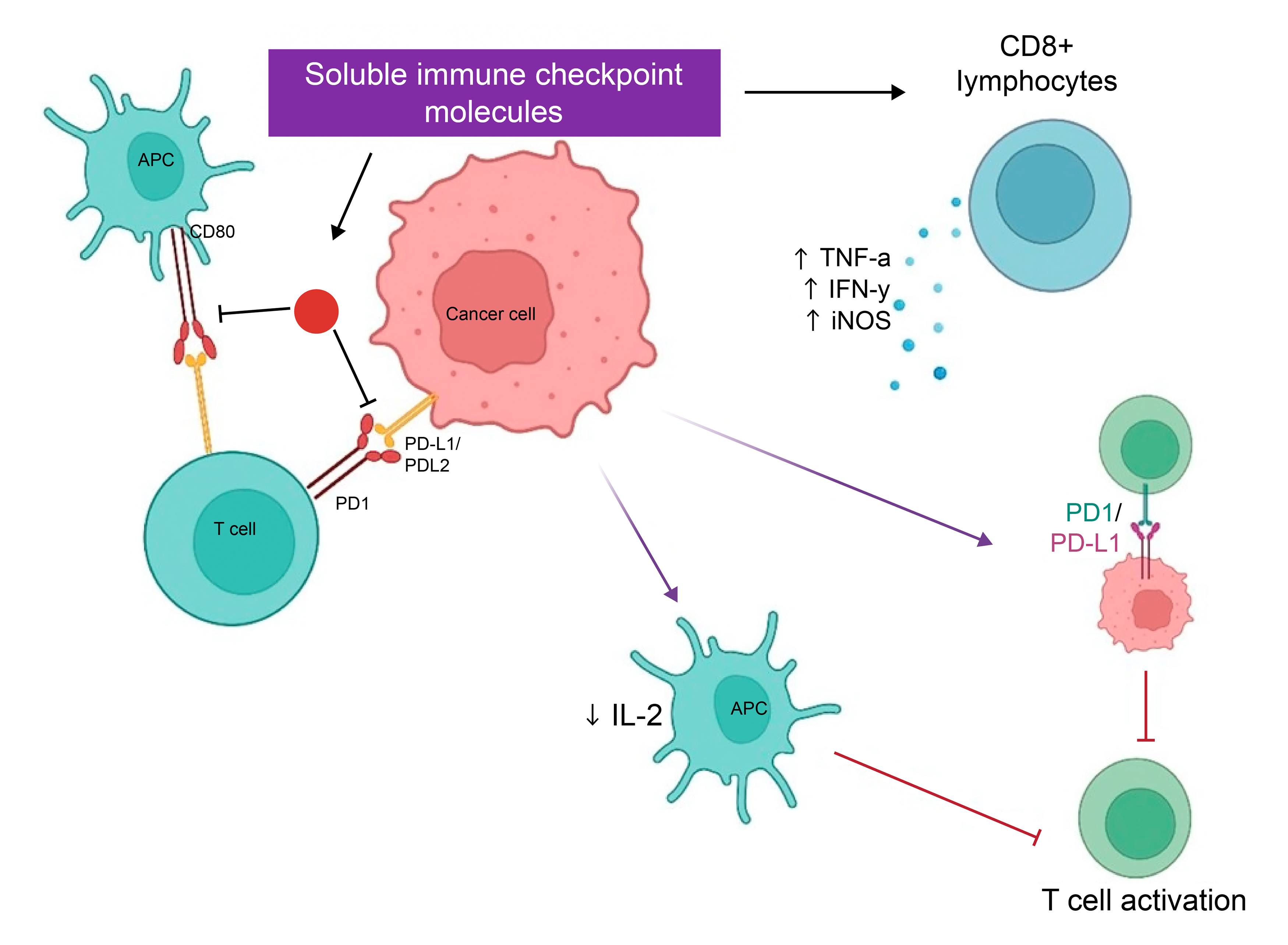
2.1. Mechanism of Action of Soluble PD-1 Programmed cell death-1 (PD-1, CD279) is a 55-kDa transmembrane protein containing 288 amino acids, extracellular N-terminal, membrane-permeating domain and a cytoplasmic tail at N and C ends with two tyrosine bases [16]. PD-1 is expressed in activated T cells, natural killer (NK) cells, monocytes, macrophages, dendritic cells (DCs) and B lymphocytes. Mechanistically, it is an inhibitor of both adaptive and innate immune responses, and is known to be highly expressed on tumor-specific T cells [17]. With regards to its role in the immune microenvironment, it is described to have both protective and harmful roles. On one hand, it plays a significant role in the regulation/maintenance of immune tolerance to prevent host tissue damage while, on the other hand, it facilitates tumor immune escape via its overexpression [18]. With respect to soluble PD-1 (sPD-1), it is generated via proteolytic cleavage of membrane-bound PD-1. Soluble PD-1 primarily exists in two forms: one form is completely homologous with membrane-bound PD-1, while others are the spliced-out parts of membrane-bound form [19]. With regards to its role in immune microenvironment, studies have shown that sPD-1 functions as a blocker of PD-1 ligands and as such suppresses the interaction of membrane-bound PD-1 with immune checkpoint ligands and receptors, PD-L1, PD-L2 and CD80 respectively [20]. This interaction leads to several immune modulatory effects. On one hand, it can lead to enhancement of the anti-tumor response via early activation of CD8+ lymphocytes, boost lytic activity of macrophages, up regulate TNF-α, IFN-γ, inducible nitric oxide synthase (iNOS) production, reduce OX40 and IL-10 expression and block PD-L1expression on tumor cells [21]. On the other hand, it confers immune inhibitory effects such as reverse signaling effect on dendritic cells via the PD-L1/PD-L2 pathway, subsequently leading to a reduction in DC maturation, and inhibition of IL-2 production and T cell activation [22]. 2.2. Role of Soluble PD-1 as a Biomarker in NSCLC Patients Treated with Immunotherapy Several studies have reported on the role of soluble PD-1 as a dynamic biomarker in immunotherapy treated NSCLC patients. Soluble forms of PD-L1 (sPD-L1), such as secreted splice variants (secPD-L1) and exosomal PD-L1 (exo-PD-L1), have recently been found in peripheral blood and have been shown to suppress T cell functions, allow tumor evasion, and accelerate tumor growth [23]. A study including 189 NSCLC patients monitored levels of soluble PD-L1 (sPD-L1) and soluble PD-1 (sPD-1) before and after starting ICI therapy. The results showed improved overall survival (OS) (hazard ratio 0.24, p value: 0.037) and high levels of sPD-1 for patients after receiving anti-PD-1 monotherapy. On the other hand, patients receiving combination of ICI and chemotherapy, no significant change in expression levels were seen, suggesting that sPD-1 can be a potential predictive response biomarker on ICI monotherapy [24]. Similarly, another study on 13 NSCLC patients, that measured change in levels of sPD-1 before and after anti-PD-1 treatment showed that increased expression of sPD-1 is significantly correlated to disease progression (p = 0.024) [14]. Another study, measuring the change in the expression of sPD-1 before anti-PD-1 (Nivolumab) treatment and after two cycles (post 28 days) in 51 NSCLC patients reported that patients with >30% increase in sPD-1 level, after two cycles of nivolumab, had a better progression-free and overall survival as compared to those with decreased levels of sPD-1 [25]. Similarly, a study on 22 NSCLC patients, investigating the role of soluble PD-1 on treatment dynamics showed comparable results to the above-mentioned studies. The level of soluble PD-1 before anti-PD-1 treatment and at the time of first clinical evaluation (after 3 months of treatment) showed dynamic modulation. A significant decrease during Nivolumab treatment was found to be associated with clinical response while increase was associated with non-response to treatment [26]. A study examined the predictive significance of baseline and dynamic changes in soluble PD-1 (sPD-1) and soluble PD-L1 (sPD-L1) levels concerning treatment outcomes. The positivity of either sPD-L1 or sPD-1 was used to calculate a composite score known as sCombo. The baseline positive of this score was substantially linked with shorter progression-free survival (PFS) of 78 days (95% CI: 55–109) compared to 658 days (95% CI: 222-not achieved) in score-negative individuals (p-value = 0.0002). Overall survival (OS) was also worse for individuals with a positive score, with a hazard ratio (HR) of 3.99 (95% CI: 1.63–9.80, p = 0.003). In multivariate analysis, sCombo score positive continued to be an independent predictor of shorter PFS, with an HR of 2.66 (95% CI: 1.17–6.08, p = 0.02) [27]. Though, limited studies have been performed on sPD-1 profiling in immunotherapy-treated NSCLC patients, results from published literature indicate a trend and scope of sPD-1 kinetics with anti-PD-1 treatment dynamics, indicating its significance as a prognostic and predictive biomarker.
3.1. Mechanism of Action of Soluble PD-L1 Programmed Death Ligand-1 (PD-L1, CD274) is a 40 kDa type I transmembrane glycoprotein that can exist as multiple splice variants, PD-L1 lncRNA splice isoforms and truncated PD-L1 [28]. Two possible alternative splicing events-either at the mid-exon or alternative polyadenylation led to the removal of the exon encoding the PD-L1 transmembrane domain in the CD274 transcript, thus, leading to the generation of soluble PD-L1 [28,29]. Studies have documented that sPD-L1 can only be generated by tumor cells and activated mature DCs [28]. Though the exact mechanism of action of soluble PD-L1 in the immune microenvironment is still unclear, studies have documented various roles of this mediator in immune regulatory processes. For instance, sPD-L1 has been reported to induce negative regulation of T-cell function by binding to PD-1. In addition to this, it has been documented that specific interaction between sPD-1/sPD-L1 may alter the activity of immune cells, leading to immune suppression via the reduction in TH1 cytokines and increased TH2 cytokines expression [30,31]. Moreover, it has been reported that a unique variant of sPD-L1, consisting of a unique18 aa tail can homodimerize and induce immunosuppressive activity via T-cell apoptosis, inhibition of lymphocyte function, T-cell proliferation, and IFN-gamma production [32]. However, studies have reported that sPD-L1 lacks measurable T-cell inhibitory activity but rather functions as a receptor antagonist by blocking the inhibitory activity of membrane-bound PD-L1 [28]. 3.2. Role of Soluble PD-L1 as a Biomarker in NSCLC Patients Treated with Immunotherapy Studies investigating the role of PD-L1 as a prognostic and predictive biomarker have shown interesting results. For instance, a study investigating the role of pre-treatment sPD-L1 levels in 233 NSCLC patients treated with anti-PD-1 (Nivolumab) found that high pre-treatment sPD-L1 levels were significantly associated with a lower disease control rate and poorer progression-free, and overall survival [33]. Similarly, a study on the impact of pre-treatment sPD-L1 levels on anti-PD-1 treatment dynamics/clinical outcomes was assessed in 39 NSCLC patients. The study reported that increased pre-treatment sPD-L1 levels confer poor response and survival in ICI-treated patients, indicating a prognostic role of this biomarker in NSCLC [34]. On the other hand, several studies have reported the kinetics of this biomarker during immunotherapy indicating its role as a dynamic marker. A study of 189 NSCLC patients determined the change in the expression level of soluble PD-L1 (sPD-L1) prior to and after immune checkpoint inhibitor (ICI) therapy. Higher baseline sPD-L1 was associated with poor progression-free survival (PFS) and OS in patients receiving ICI monotherapy, with HR of 15.4 (p = 0.009) and 11.4 (p = 0.007), respectively. Baseline sPD-L1 levels were linked with other soluble components produced by zinc-binding proteases ADAM10/17 [24]. According to a study, individuals with advanced NSCLC who exhibit elevated levels of soluble PD-L1 (sPD-L1) are more likely to have unfavorable outcomes with immunotherapy. Researchers analyzed 120 patients and discovered that those with disease control had longer PFS and OS compared to those with high sPD-L1 levels, as well as significantly lower sPD-L1 concentrations (p = 0.0006). The median PFS was 5.8 months vs. 2.5 months (HR = 0.6021, p = 0.0156), and the median OS was 16.5 months vs. 7 months (HR = 0.5354, p = 0.0071). The levels of sPD-L1 and tumor PD-L1 expression did not significantly correlate. Further, the study’s conclusion emphasizes sPD-L1’s potential for treatment guidance by showing that it is a useful marker for predicting resistance to anti-PD-1 or anti-PD-L1 therapy [35]. Another study by Costantini et al. investigated sPD-L1 levels in the plasma at three time points; at the time of diagnosis, at the initiation of nivolumab, and after 2 months (first tumor evaluation). The study found that an increase in sPD-L1 levels from baseline to the first tumor evaluation was associated with poorer progression-free survival (PFS) and overall survival (OS) [36]. Interestingly, the authors did not see any correlation between sPD-L1 concentration with tissue PD-L1, indicating that sPD-L1 can serve as an additional biomarker (in combination with tissue PD-L1) for treatment monitoring. Another study on sPD-L1 levels in 22 NSCLC patients before nivolumab treatment and after the first clinical evaluation (after 3 months of treatment) reported dynamic modulation of sPD-L1 levels with significantly higher levels observed in non-responding patients [26]. The pharmacodynamics of anti-PD-1 treatment and sPD-L1 were further observed in a large case control study. In 51 nivolumab-treated patients, plasma PD-L1 was assessed at the time of initiation of treatment and after 28 days of treatment. The results showed that the reduction in sPD-L1 levels after 28 days was associated with poor prognosis [25]. In addition to anti-PD-1 monotherapy, the role of sPD-L1 in combination therapy (chemotherapy in combination with immunotherapy) has also been investigated. A clinical trial by Bonomi et al. investigated the role of sPD-L1 in two randomized arms of patients: one arm with 10 patients on pembrolizumab monotherapy, and another arm with 10 patients on pembrolizumab combination with carboplatin and paclitaxel. Immune cell phenotyping to assess sPD-L1 levels in patients prior to treatment and at weeks 4 and 7. In both arms, increased baseline levels of sPD-L1 were associated with disease progression [37]. Another study conducted an open-label, randomized phase II trial encompassing three arms. Pembrolizumab was given to 7 patients for four cycles in Arm A, then four cycles of nab-paclitaxel in a sequential treatment regimen. Another 7 patients in Arm B followed a comparable sequential regimen, starting with four cycles of nab-paclitaxel followed by four cycles of pembrolizumab. Pembrolizumab and nab-paclitaxel were given concurrently to 6 patients in Arm C for four cycles of therapy. PFS was 10.1, 8.4, and 10.2 months for Arm A, B and C, respectively. Due to the approval of PD-1 blocking as the preferred first-line therapy, the trial was prematurely discontinued. However, it was shown that the administration of chemoimmunotherapy was successfully endured and capable of establishing long-term disease management in a particular subset of patients, in line with subsequent findings [38]. However, this is the only study in such a cohort, and larger studies on this aspect will be able to provide a better understanding of the kinetics of sPD-L1 in combination immunotherapy and its role as a biomarker of response. Though limited studies have been performed on soluble PD-L1, the results of the studies are comparable with clear attribution of sPD-L1 in poor prognosis, indicating its role as a prognostic and predictive biomarker in immunotherapy-treated NSCLC patients.
4.1. Mechanism of Action of Soluble PD-L2 PD-L2 (CD273) is a 273-amino acid long-cell surface receptor, consisting of an immunoglobulin-like variable domain (Ig-V) and an immunoglobulin-like constant domain (Ig-C) in the extracellular region, a transmembrane domain, and a cytoplasmic domain [39]. Its expression is mainly found on dendritic cells, macrophages, and sometimes in T helper cell subsets and cytotoxic T cells [40]. PD-L2 serves as the second ligand for PD-1 and is therefore involved in regulation of T cell activity. Studies report contrasting role of PD-L2 with respect to immune regulation. On one hand, PD-L2 has been documented to function as an inhibitory receptor with its interaction with PD-1, leading to decreased cytokine production by CD4+T cells and inhibition of T cell receptor (TCR)-mediated proliferation [39]. Furthermore, some studies have reported its role in the activation of T cells via IL-12 production and reverse signaling in dendritic cells [41]. Though both PD-L1 and PD-L2 serve as ligands for PD-1 with similar influence on immune regulation, structural differences exist between both which affect their binding affinity. Studies have documented structural differences of amino acids homologous to their binding sites (in PD-L2, alanine exists at position 110 while tryptophan exists at position 121). In addition to this, PD-L2 contains N64PD-L2 glycan, which is absent in PD-L1. It is postulated that this structural change affects the “snug-fit” interaction of PD-L2 with PD-1, thus, weakening its binding affinity. However, PD-L2 carries a C–D latch region (which is missing in PD-L1), that facilitates the extension of PD-L2 contact area with PD-1. Therefore, the presence of this latch helps to counterbalance the weakness brought on by alanine and N64 glycan and instead increases its binding affinity for PD-1, approximately 3-4-fold higher than PD-L1 [42,43,44,45]. Studies have reported that soluble PD-L2 is generated due to a splicing event involving a frameshift of exon 3 to an alternative acceptor site 5 base pairs downstream of the canonical acceptor site. As a result, the generated soluble PD-L2 lacks the transmembrane domain but retains the IgV-like domain. It should be noted that since no deletion of the exon encoding transmembrane domain occurs during the splicing event, it is postulated that soluble PD-L2 can play a role in immune regulation due to its Ig-V domain and the existing exon for transmembrane region thus facilitating its binding with PD-1 [46]. 4.2. Role of Soluble PD-L2 as a Biomarker in NSCLC Patients Treated with Immunotherapy The role of soluble PD-L2 as a biomarker in immunotherapy-treated NSCLC patients has been reported in limited studies. A study on 22 NSCLC patients treated with Nivolumab assessed the role of soluble mediators in treatment response. Changes in the kinetics of sPD-L2 before initiation of treatment and after 6 cycles (3 months’ post-treatment) of Nivolumab showed interesting results. Dynamic modulation of sPD-L2 was observed, with levels of sPD-L2 significantly decreasing during nivolumab treatment in responding patients (though non-significantly). However, a combination of decreased levels of sPD-L2 with soluble mediators PD-L1, CD137, Tim-3, and BTLA-4 during treatment was significantly associated with a favorable clinical response. This suggests that these soluble markers may work synergistically to modulate the tumor microenvironment. Interestingly, the authors also found a prognostic value of sL2. Low levels of sPD-L2 before treatment initiation were significantly associated with longer clinical response indicating the prognostic value of pre-treatment sPD-L2 as a biomarker of response [26]. Another research investigated the potential relevance of sPD-L2, sPD-L1, sIL-2, sIFN-γ, granzyme B and microRNAs as indicators of ICI responsiveness among 43 NSCLC patients treated with Nivolumab, the results of sPD-L2 demonstrated no association with clinical outcomes. Additionally, grade 3–4 toxicities were linked to low sPD-L2, low sIL-2, and high sIFN-γ [47]. Another study on 43 patients in Nivolumab-treated NSCLC patients aimed to assess the role of sPD-L2 as a predictive biomarker. Plasma sPD-L2 levels were assessed at diagnosis, at the start of treatment and after 2 months (first tumor evaluation). It was observed that, though sPD-L2, was not associated with progression-free or overall survival, lower levels of sPD-L2 at diagnosis and start of treatment were significantly associated with grade 3–4 adverse events. This suggests a dynamic role for this biomarker in predicting immune-related adverse events [36].
5.1. Mechanism of Action of Soluble CTLA-4 CTLA-4, binds to CD80 and CD86 ligands present on APCs and regulates the immune system. The second signal needed for complete T cell activation and proliferation is blocked by this interaction between the ligands and the CD28 receptor on T cells. Through efficient inhibition of T cell activation caused by the blockage of the CD28-CD80/CD86 interaction, CTLA-4 produces an immunosuppressive effect [48]. This suppression can be harmful in the setting of cancer by impeding the body’s capacity to generate an efficient anti-tumor response, but it is advantageous in avoiding hyperactive immune responses and autoimmunity [49]. The method by which CTLA-4 controls immunological responses demonstrates its dual function in immune regulation. On the one hand, it promotes immunological homeostasis and the prevention of autoimmune disorders by suppressing T cell activation [50]. However, in the case of cancer, this same technique can be detrimental since it reduces the immune system’s capacity to efficiently fight tumor cells. This complicated role of sCTLA-4 emphasizes the need for more study to better understand its activities and create ways to modulate its levels, thereby improving treatment outcomes in cancer patients [51]. In addition to being expressed on the cell surface, the CTLA-4 protein is also found in soluble form as soluble CTLA-4 (sCTLA-4), which was initially discovered by Magistrelli et al. Non-activated T cells release the fully functional 137-amino-acid polypeptide sCTLA-4, which is also seen in normal human serum. Extracellular, transmembrane, and cytoplasmic sections make up mature CTLA-4 molecules. Although having no transmembrane domain, sCTLA-4 secretes from cells while maintaining its amino acid residues that bind to B7 [52]. Although sCTLA-4 lacks the transmembrane domain, which allows cell secretion, mature CTLA-4 has extracellular, transmembrane, and cytoplasmic sections. Additionally, in sCTLA-4, the amino acid residues that bind to B7 remain unchanged [53]. In vitro research on human T cells has demonstrated that sCTLA-4 release peaks throughout immunological responses and has a potent inhibitory effect. In particular, when sCTLA-4 is blocked, antigen-driven proliferation and cytokine release are markedly enhanced [54]. Recent studies indicate that solublesCTLA-4 has a significant down-regulatory influence on the immune response by preferentially binding to B7 molecules on antigen-presenting cells (APCs), as opposed to CD28 on T lymphocytes [52]. In cancer patients, notably individuals with NSCLC, increased levels of sCTLA-4 have been linked to poorer clinical results when treated with ICIs [55]. According to studies, high plasma levels of sCTLA-4 are associated with a lack of reactivity to therapy such as anti-PD-1 medications [56]. This shows that sCTLA-4 might serve as a predictive biomarker, assisting in identifying individuals who are less likely to benefit from ICI therapy and possibly driving more tailored treatment methods [57]. 5.2. Role of Soluble CTLA-4 as a Biomarker in NSCLC Patients Treated with Immunotherapy sCTLA-4 is a potential biomarker for determining the effectiveness of immunotherapy in patients with NSCLC. Recent research has investigated its ability to predict treatment response and correlate with clinical outcomes [57]. For example, a study conducted by Hayashi et al. [51] investigated the role of sCTLA-4 as a biomarker in 50 individuals with NSCLC who received nivolumab. Higher baseline levels of sCTLA-4 were related to worse progression-free survival, indicating a negative relationship between sCTLA-4 levels and the therapeutic response. Patients with high sCTLA-4 levels had a poorer response to nivolumab. The study found a significant p-value (<0.05), highlighting the strength of the correlation [51]. A study of 536 patients with primary resected stage I–IIIA NSCLC evaluated the immunohistochemistry expression of CTLA-4 in the tumor and stromal tissues of the main tumors, as well as the locoregional metastatic lymph nodes. The study discovered that CTLA-4 expression in primary tumors’ tumor epithelial cells (T-CTLA-4) and stromal cells (S-CTLA-4) were not correlated with each patient’s disease-specific survival (DSS). In the squamous cell carcinoma subgroup, however, increased sCTLA-4 expression predicted significantly better DSS (HR 0.62, 95% CI 0.41–0.93, p = 0.021). On the other hand, there was a negative predictive effect associated with T-CTLA-4 expression in metastatic lymph nodes (HR 1.65, 95% CI 1.03–2.65, p = 0.039). These results reveal significant variations in respective tumor microenvironments and imply that CTLA-4 expression has different prognostic consequences in original tumors vs. metastatic NSCLC lymph nodes [58].
6.1. Mechanism of Action of Soluble LAG-3 Lymphocyte-activation gene 3 (LAG-3) or CD223 is an immunological checkpoint receptor that regulates immune responses, namely self-tolerance and autoimmunity. LAG-3 is expressed on a variety of cell types, including CD4+ and CD8+ T cells, as well as Tregs, and is necessary for proper T cell regulation and homeostasis [59]. While LAG-3 is often investigated in its membrane-bound variant on the surface of T cells, sLAG-3 may also be found in the extracellular environment. The mechanism of action for soluble LAG-3 differs from that of its membrane-bound counterpart and includes numerous critical roles [60]. LAG-3 is thought to regulate the immune response by attaching MHC-II molecules to APCs. This connection can alter the activation and function of many immune cells, including APCs, dendritic cells, and T-cells, and hence affect the overall immune response [59]. LAG-3 suppresses the proliferation and activation of T cells by binding to MHC-II on APCs, and this helps to prevent the immune system from becoming overactive, which can result in autoimmunity or chronic inflammation [61]. LAG-3 plays a major role in stimulating the development of regulatory T cells (Tregs), which helps reduce exaggerated immune responses and promotes immunological tolerance and homeostasis [62]. Additionally, LAG-3 interacts with immune checkpoint molecules such as CTLA-4 and PD-1 to form a complex regulatory network that modulates the immune response. These interactions can strengthen the inhibitory signals produced by these checkpoints, further suppressing the immune response. The interaction of several checkpoints demonstrates the complexity of immune regulation and the potential for addressing these pathways in therapeutic procedures [63]. sLAG3, a soluble form of LAG3, is formed by alternative splicing and proteolytic cleavage of the extracellular domain and is mostly secreted by dendritic cells (DCs), but also by B and T lymphocytes, and its function is largely unknown. Some studies suggest its involvement as a costimulatory molecule by binding to MHC-II and encouraging DC maturation; however, these are largely connected to synthetic sLAG3-Ig proteins employed in therapeutics, which have a high MHC-II affinity compared to human sLAG3 [64]. However, human sLAG3 has been postulated to function as a co-inhibitor of immune responses by decreasing monocyte development and antigen presentation capabilities, inhibiting their activation of T-cell proliferation [65]. 6.2. Role of Soluble LAG-3 as a Biomarker in NSCLC Patients Treated with Immunotherapy sLAG-3 has emerged as a promising biomarker in NSCLC, providing insights into tumor immune suppression and treatment response by reflecting the dynamic interaction of immunological checkpoints and tumor growth [63]. A study examined soluble lymphocyte activation gene-3 (sLAG-3) and CD4/CD8 ratio dynamics as biomarkers for predicting outcomes in advanced solid cancer patients following immune checkpoint inhibitor (ICI) treatment. Higher pre-treatment sLAG-3 levels were linked with poor progression-free (HR: 1.005, p = 0.039) and overall survival (HR: 1.006, p = 0.015) outcomes, but a lowering CD4/CD8 ratio following therapy was associated with improved overall survival (p = 0.012, HR: 3.32). A combined immunological score that included sLAG-3 levels and CD4/CD8 ratio dynamics had the highest predictive potential for survival (HR: 10.3), indicating that they might be used as robust biomarkers to guide ICI treatment [64]. Another study looked at the role of soluble LAG-3 (sLAG-3) as a biomarker in non-small cell lung cancer (NSCLC) and how it is associated with the disease stage. Serum sLAG-3 levels were evaluated using enzyme-linked immunosorbent assay (ELISA) in 247 individuals, comprising 71 with benign illnesses and 176 with NSCLC. The study discovered that sLAG-3 levels were significantly higher in early-stage NSCLC (stage I-II) compared to later stages (stage III-IV, p < 0.001). This suggests that lower sLAG-3 expression in advanced NSCLC may indicate a weaker immune response. The findings emphasize the possibility of boosting sLAG-3 levels as a treatment option for advanced NSCLC [66].
7.1. Mechanism of Action of Exosomes The endosomal compartment of most eukaryotic cells produces membrane extracellular vesicles, or exosomes, typically ranging in size from 30 to 150 nanometers. A diverse range of biological elements, including proteins, short RNAs, lipids, deoxyribonucleic acids (DNAs), messenger RNAs (mRNAs), and more, are found within the lipid bilayer structure that makes up exosomes. By transmitting these useful proteins, these extracellular vehicles (EVs) fulfill their role as mediators of intercellular communication [67,68]. Specifically, exosomes are actively released by cancer cells into the circulation, where they travel throughout the body and become a component of the intricate communication network that is supported by the tumor microenvironment. These exosomes can promote cellular communication and function as non-invasive cancer indicators. By interacting with the immune cell protein PD-1, this substance can aggressively inhibit CD8+ cells. Exosomes can also prevent monocyte differentiation, decrease NK cell function, and transform myeloid precursor cells into myeloid suppressor cells. In vitro studies have demonstrated that elevated levels of exosomal PD-L1 can inhibit T cell activation, even though the role of exosomal PD-L1 in vivo is still unknown. They are often found in a variety of physiological fluids. Exosomal-PD-L1 is noticeably detectable in human plasma and has the potential to become a crucial biomarker [69,70]. 7.2. Role of Exosomal PD-L1 as a Biomarker in NSCLC Patients Treated with Immunotherapy Studies have investigated the relationship between the expression of PD-L1 mRNA in exosomes obtained from plasma and the responsiveness of anti-PD-1 antibodies in melanoma and NSCLC. For example, a study was conducted by Del Re et al. (2018) [71] on 8 patients with non-small-cell lung cancer (NSCLC) where 2 individuals had a partial response (PR), 2 had stable disease (SD), and 4 had progressive disease (PD). The study demonstrated that the response to anti-PD-1 antibodies in people with melanoma and non-small cell lung cancer (NSCLC) was strongly correlated with the presence of PD-L1 mRNA within exosomes extracted from plasma. The tumor response was correlated with the number of copies of PD-L1 mRNA per milliliter. number of copies of PD-L1 mRNA per milliliter was related to tumor response. Patients with PD showed a considerable increase (mean: 295 to 550 copies per mL, from baseline to 2 months), but those who achieved PR showed a decrease (mean: 1015 to 300 copies per mL). Patients with SD showed a moderate change (mean: 455 to 285 copies per mL). 231 ± 55.7, p = 0.012). There was a noticeable variation in the number of PD-L1 mRNA copies per milliliter in the entire research cohort. This difference was statistically significant in with progressing disease (204 vs. 416, baseline vs. 2 months later, p = 0.001) and patients exhibiting complete or partial responses (830 at baseline compared to 242 after 2 months, p = 0.016). The difference between patients with stable disease (298 at baseline vs. 247 after 2 months, p = 0.586) was not as noticeable as expected. It must be noted that, at first, people who had complete and partial responses had much more PD-L1 mRNA copies per milliliter than people who had stable disease or who were progressing from their disease (830 vs. 231 p = 0.012) [71]. In a trial of 17 patients with advanced or metastatic NSCLC, researchers investigated the possibility of serum-derived exosomal PD-L1 (exoPD-L1), exoPD-L2, and exoPD-1 as biomarkers for monitoring immunotherapy responses. The study discovered that exoPD-L1 levels in patients were substantially greater than in their soluble forms and healthy controls. Importantly, a drop in exoPD-L1 levels was related to better treatment outcomes, whereas an increase was connected to tumor growth. The study indicated that exosomal biomarkers may be more successful than standard tissue biopsies in predicting and monitoring treatment results in NSCLC patients undergoing immunotherapy [72]. In another study, the clinical significance of Exo-PD-L1, sPDL1, and the overall PD-L1 profile was investigated in 65 stage I-IIIA NSCLC patients, who had not previously received therapeutic procedures such as chemotherapy, targeted therapy, surgical removal, or radiation. The results showed a concentration of 1.84 pg/mg exosomal PD-L1 protein concentration in healthy controls, whereas in patients with stage I/II and III/IV the levels were 3.02–1.67 pg/mg and 5.17–3.16 pg/mg respectively. In healthy controls the level of Exo-PD-L1 was low when compared to affected individuals. Furthermore, large tumor size and advanced disease with profound metastasis was associated with higher exo-PD-L1 indicating its potential to be used as a biomarker in NSCLC [73].
8.1. Mechanism of Action of Cytokines Cytokines, such as tumor necrosis factors (TNF), interleukins, interferons, and chemokines, as well as growth factors including TGF-β, VEGF, and EGF, can promote or inhibit tumor growth depending on external factors [74]. Cytokines, being important immune response regulators, promote inflammation and modulate several intracellular signaling pathways that govern the growth, proliferation, and migration of cells, contributing to the development as well as the advancement of cancer [75] (Table 2). Recent improvements in targeting these molecules have resulted in the creation of innovative treatment approaches, including monoclonal antibodies, bispecific antibodies, receptor inhibitors, fusion proteins, and synthetic cytokine variations [76]. These techniques seek to control the immune system, slow tumor growth, and overcome resistance to standard treatments. Combining these tailored medicines with additional therapeutic options shows promise for improving patient outcomes. Ongoing research and clinical trials are crucial for improving our understanding and implementation of cytokine- and chemokine-targeted treatments in cancer [77]. The effects of pro-inflammatory and anti-inflammatory cytokines on immune microenvironment and cellular signaling pathways. 8.2. Mechanism of Action of Pro-Inflammatory Cytokines 8.2.1. Tumor Necrosis Factor-Alpha (TNF-α) TNF-IL is a key contributor to the development of NSCLC and a known pro-inflammatory cytokine that helps in immune modulation and understanding of tumor biology. Chronic inflammation, including elevated TNF-α levels, can lead to cancer formation and progression by damaging DNA and activating inflammatory cytokines such as IL-1 and IL-6 [78]. TNF-α promotes angiogenesis through VEGF and influences immune cell recruitment, leading to an immunosuppressive environment that promotes tumor development. It also stimulates tumor cell growth and survival by activating NF-κB, which prevents apoptosis [79]. In clinical settings, elevated levels of TNF-α are associated with more advanced stages of the disease and a worse prognosis. Additionally, they can offer valuable information on the efficacy of targeted and anti-inflammatory treatments, which can inform treatment plans and patient care [80,81]. 8.2.2. Interleukin-6 (IL-6) IL-6 is a key cytokine that contributes to cancer development, particularly NSCLC, via a variety of methods. IL-6 binds to its receptor complex, consisting of IL-6Rα and the gp130 component, generating a hexametric structure that activates JAKs, namely JAK1 and JAK2 [82]. This activation causes the phosphorylation of STAT3, which subsequently translocate to the nucleus and drives the transcription of genes that increase cell proliferation, survival, and inflammation. IL-6 can activate the NF-κB pathway, leading to increased inflammation and cellular survival by promoting the production of pro-inflammatory genes [83]. IL-6 induces the liver to create acute-phase proteins, such as CRP and fibrinogen, which are indicators of systemic inflammation. IL-6 assists in tumor development and metastasis of cancerous tumors by promoting angiogenesis through VEGF activation and establishing an immunosuppressive environment [84]. In NSCLC, elevated IL-6 levels typically correspond to poor prognosis, emphasizing the potential of this molecule as a prognostic indicator and a target for novel treatment strategies that target suppressing its signaling pathways [83]. In NSCLC, elevated IL-6 levels have been associated with poor prognosis, suggesting that it could serve as a target for novel therapeutic techniques that aim to disrupt its signaling pathways as well as a prognostic indicator [85]. 8.2.3. Interleukin-1 Beta (IL-1β) IL-1β is another pro-inflammatory cytokine that regulates immunological and inflammatory responses via complex signaling pathways. It interacts with both IL-1 receptor type 1 (IL-1R1) and IL-1 receptor accessory protein (IL-1RAcP), activating the MyD88 adaptor protein [86]. Tumor necrosis factor receptor-associated factor 6 (TRAF6) is subsequently recruited and activated because of this activation, which also activates interleukin-1 receptor-associated kinases (IRAKs). TRAF6 activates the IKK complex, resulting in the degradation of IκB proteins and the release of NF-κB dimers into the nucleus. This promotes the transcription of pro-inflammatory genes [87]. Furthermore, several MAPKs are activated by IL-1β signaling, and these MAKs further induce the production of cytokines and chemokines, so promoting the inflammatory response [88]. In a systemic manner, IL-1β causes fever and activates the liver to create acute-phase proteins, which are indicators of inflammation, such as fibrinogen and C-reactive protein (CRP). These mechanisms highlight the critical function of IL-1β in both acute and chronic inflammatory conditions [89]. 8.2.4. Interleukin-8 (IL-8) CXCL8, another name for IL-8, is a crucial chemokine that plays an essential role in the proinflammatory response by activating and recruiting neutrophils. GPCRs expressed on the surface of neutrophils and other cells, CXCR1 and CXCR2, are the receptors that IL-8 binds to cause its effects. Intracellular G-proteins are activated because of these receptors being activated by IL-8 binding [90]. This activation causes PLC to produce IP3 and DAG, which elevate intracellular calcium levels and activate a variety of signaling pathways. The activation of these pathways improves neutrophil chemotaxis, which directs neutrophils to areas of infection or inflammation, as well as their activation, including oxidative burst and degranulation [91]. IL-8 promotes endothelial cells to produce adhesion molecules such as ICAM-1 and VCAM-1, promoting neutrophil migration across the endothelium. Along with its function in acute inflammation, IL-8 is also affiliated with chronic inflammatory disorders and cancer [92]. In these conditions, it fosters tumor expansion, angiogenesis, and metastasis, all of which are associated with a poor prognosis in various cancer types. These capabilities underscore IL-8’s critical involvement in mediating and maintaining inflammatory processes under various situations [87,93]. 8.2.5. Interleukin-17 (IL-17) Interleukin-17 (IL-17) is a pro-inflammatory cytokine essential for mediating inflammation and immune responses. Produced by Th17 cells, IL-17 also functions by interacting to its receptor complex, which consists of IL-17RA and IL-17RC and is produced by other immune cells such as γδ T cells and innate lymphoid cells. Numerous cell types, such as endothelium, fibroblast, and epithelial cells, have this receptor complex on them [94]. IL-17 stimulates intracellular signaling pathways, particularly the NF-κB and MAPK pathways, when it binds to its receptor. Pro-inflammatory cytokines and chemokines are transcriptionally produced when NF-κB is activated, promoting inflammation, and attracting immune cells to infection or damage sites. In a similar vein, the inflammatory mediators’ synthesis is further enhanced by the MAPK pathway. In order to facilitate the recruitment of signaling proteins and promote these inflammatory responses, the adaptor protein Act1 is crucial to this process [95]. Biological consequences of IL-17 include cytokines such as IL-6 and IL-8 being produced and endothelial cells expressing adhesion molecules, which aids in leukocyte recruitment. Additionally, it triggers the production of peptides that are antimicrobial and other inflammatory chemicals by epithelial cells, which guard against pathogens but can lead to tissue damage in cases of chronic inflammation [96]. Furthermore, in the setting of cancer, IL-17 supports tumor development and spread by boosting the synthesis of vascular endothelial growth factor (VEGF), which in turn promotes angiogenesis [97]. Overproduction of IL-17 under pathological settings is associated with autoimmune illnesses, including multiple sclerosis, psoriasis, and rheumatoid arthritis, where it causes tissue damage and persistent inflammation [98]. It has a complicated role in cancer; although it can stimulate tumor development through angiogenesis and inflammation, research is still being done on how it affects antitumor immunity. Knowing the processes behind IL-17’s dual functions in disease pathology and protective immunity offers important insights into the protein’s potential as a target for therapy [99]. 8.3. Mechanism of Action of Anti-Inflammatory Cytokines 8.3.1. Interleukin-10 (IL-10) IL-10, an anti-inflammatory cytokine, regulates immune responses by attaching to its receptors IL-10Rα and IL-10Rβ on immune cells such as macrophages, dendritic cells, and T cells. This interaction promotes JAK1 and Tyk2, which phosphorylate and activate STAT3, causing it to translocate into the nucleus and regulate anti-inflammatory gene expression [100]. IL-10 decreases the synthesis of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β by inhibiting the NF-κB and MAPK pathways, decreasing damage to the tissues. However, under some circumstances, IL-10 might also have immunostimulant properties [101]. Considering IL-10’s critical function in immune regulation, conditions marked by hyperinflammatory states, like cancer, or infectious disorders, like COVID-19 and post- COVID-19 syndrome, may be affected by this cytokine [102]. Furthermore, IL-10 regulates macrophage and dendritic cell activities, reducing the expression of activation markers and the formation of reactive oxygen species. Additionally, it affects T cell responses by promoting the growth of regulatory T cells and preventing Th1 and Th2 cell differentiation. Due to these characteristics, IL-10 plays a vital role in regulating inflammatory reactions and holds therapeutic promise for the treatment of autoimmune disorders and some types of cancer [87,103]. 8.4. Transforming Growth Factor-Beta (TGF-β) TGF-β is a multifunctional cytokine that is essential for controlling tissue healing, cell division, and immunological responses. TGF-β binds to its receptor complex, which consists of TGF-β receptor types I and II (TGFBR1), on target cells to produce its effects [104]. SMAD proteins, especially SMAD2 and SMAD3, are phosphorylated and activated through the activation of receptor serine/threonine kinases resulting from this interaction. The phosphorylated SMAD2/3 forms a complex with SMAD4 that translocates into the nucleus and affects the transcription of genes involved in immunological modulation, cell proliferation, and differentiation [105]. By stimulating the formation of regulatory T cells (Tregs) and reducing the generation of pro-inflammatory cytokines, TGF-β prevents the activation and proliferation of immune cells, including T cells and B cells. Subsequently, it also affects macrophage and dendritic cell function, diminishing their capacity to trigger T-cell responses and inflammatory cytokine production [106]. TGF-β plays a role in immune regulation, fibrosis, and cancer progression. It promotes EMT, increases extracellular matrix production, and creates an immunosuppressive microenvironment, facilitating tumor progression. TGF-β has a critical part in both normal and pathological processes, as evidenced by its various roles [107]. 8.5. Role of Pro and Anti-Inflammatory Cytokines as Biomarkers in NSCLC Patients Treated with Immunotherapy In a study, serum-derived exosomes from 17 NSCLC receiving ICI treatment were assessed for TNF-alpha levels. According to the research, TNF-alpha was increased in responding patients, and this was linked to a considerable rise in exosomal PD-1 (p = 0.0156) and IFN-γ (p = 0.0156). TNF-alpha may be a sign of immune system activation and treatment response, while non-responding individuals showed a distinct cytokine profile. These findings highlight the potential of TNF-alpha as a useful biomarker for evaluating and forecasting the effectiveness of ICI treatment in patients with NSCLC [72]. A study of 29 stage IV NSCLC patients receiving single-agent PD-1 inhibitors showed high baseline levels of inflammatory markers. The results were associated with worse PFS, with p-values < 0.001. Increased IFN-γ levels were linked to improved PFS (p < 0.001). Long-term responders (PFS > 30 weeks) showed decreased IL-6, NLR, and SII levels, but greater IFN-γ levels (all p-values < 0.001). Inflammatory indicators increased throughout time, indicating that the condition was progressing. There was no significant correlation between these indicators and irAEs [108]. According to another study, cytokine levels in untreated advanced NSCLC patients were higher than in healthy controls. Chemotherapy was shown to reduce these levels, whereas immunotherapy alone did not. With hazard ratios of 0.28–0.34 for OS and 0.42–0.54 for PFS lower baseline TNF-RI levels were linked to improved survival outcomes. P-values ranged from 0.014 to 0.001. irAEs were associated with higher baseline levels of IL-1β and angiogenin, with fold increases ranging from 7–9 and p-values from 0.009 to 0.0002. Nonetheless, neither the course of the disease nor its clinical prognosis was predicted by alterations in cytokine levels after therapy. irAEs and treatment benefits may be predicted with baseline cytokine levels, but long-term illness monitoring is not possible with these measurements [109]. In a study of 88 participants (53 NSCLC patients, 17 with non-malignant lung diseases, and 18 healthy volunteers), researchers discovered that NSCLC patients had a higher number of CD4(+) CD25(+) FoxP3(+) regulatory T (Treg) cells with increased expressions of immunosuppressive molecules such as CTLA-4, LAG-3, and PD-1. Intratumorally Treg cells had the greatest quantities of these co-inhibitory compounds, indicating a potent immunosuppressive action. Additionally, the number of Treg cells and their functional molecules increased as cancer progressed. NSCLC patients had greater plasma levels of TGF-β, and IL-10 compared to healthy controls, indicating the importance of Treg cells in tumor microenvironments [110]. Another study of 60 NSCLC stage III/IV patients treated with anti-PD-1-based chemotherapy found that greater on-treatment IL-1β and IFN-γ levels correlated with better outcomes, including enhanced response and extended PFS (p = 0.013 and p = 0.007, respectively). In contrast, higher on-treatment IL-6 levels were associated with a worse response, shorter PFS, and OS (p = 0.012, p = 0.020, and p = 0.037, respectively). High pretreatment IL-2 levels indicated longer PFS (p = 0.049), but high IL-8 levels predicted shorter OS (p = 0.006) [111]. In addition, a trial of 102 NSCLC patients undergoing immunotherapy showed how increased CXCL12 levels were linked with significantly poorer outcomes, including shorter OS (12.20 vs. 44.84 months, p = 0.008) and PFS (3.76 vs. 14.40 months, p < 0.001). Patients with PD-L1 positive had superior OS (44.84 vs. 20.42 months, p = 0.087) and ORR (70.0% vs. 28.8%, p < 0.001). PD-L1 positive patients exhibited a significantly longer median PFS (25.35 vs. 4.64 months, p = 0.003). Patients with high CXCL12 levels and low PD-L1 expression had the worst results, with a lower ORR (27.3% vs. 73.7%, p < 0.001) and shorter PFS (2.44 vs. 25.35 months, p < 0.001). Combining PD-L1 and CXCL12 levels has a higher predictive value for clinical outcomes. By enabling customized therapies based on individual biomarker profiles, the use of cytokines, chemokines, and new interleukins as cancer biomarkers has the potential to transform personalized medicine by increasing therapeutic efficacy and reducing adverse effects. Strict clinical verification and standardized testing procedures are necessary to guarantee these biomarkers’ dependability [112]. Enhancing treatment methods and providing a full knowledge of cancer biology can be achieved by integrating data on cytokines and chemokines with genomes, proteomics, and metabolomics. Technological developments like liquid biopsies and high-throughput tests will enhance biomarker sensitivity and non-invasive monitoring, enabling more accurate tracking of cancer growth and treatment outcomes [113]. Management of patients and customized therapy planning will be improved by incorporating cytokine and chemokine data into clinical decision support systems. For novel biomarkers to be clinically adopted, it is essential that they satisfy regulatory requirements and that ethical issues be addressed. Lastly, global collaboration in research and the involvement of varied populations will speed the creation and validation of novel biomarkers, ensuring widespread use and efficacy [112].
Pro-Inflammatory
CytokineEffect on Immune Microenvironment
TNF-α
-Receptor Binding of NF-α to TNF receptors (TNFR1 and TNFR2) on various cells
-NF-κB and MAPK pathways activated
-Increase in the production of pro-inflammatory cytokines and chemokines
-Apoptosis in cells
IL-6
-IL-6 binds to gp130 and IL-6Rα
-JAK1 and JAK2 are activated
-STAT3 Activation
-Activation of the NF-κB pathway
-Stimulates the synthesis of fibrinogen and CRP
-Immune suppression, can be anti-inflammatory under some conditions
IL-1β
-Binding of IL-1β to IL-1R1 and IL-1RAcP
-Activation of MyD88
-IKK is stimulated by TRAF6 and releases NF-κB into the nucleus
-Pro-inflammatory genes are transcriptionally stimulated by NF-κB
-IL-1β triggers the activation of MAPKs
-Rise in the production of chemokines and cytokines
IL-8
-Binding of IL-8 to CXCR1 and CXCR2 on neutrophils.
-GPCR activation
-G-Protein Activation
-PLC production of IP3 and DAG
-Increasing intracellular calcium levels and activating signaling pathways
-Neutrophil Chemotaxis
-Stimulates adhesion molecules ICAM-1, VCAM-1 production
IL-17
-Binding of IL-17 to its receptor complex, IL-17RA and IL-17RC
-Activation of intracellular signaling pathways
-Phosphorylation and degradation of IκB
-NF-κB dimers (such as p65/p50) released
-Translocation of NF-kB to the nucleus
-Activation of MAPK pathway
-Recruitment of signaling molecules by Act1
-Increase in other proinflammatory cytokines
IL-10
-Receptor Binding of IL-10 to IL-10Rα and IL-10Rβ on immune cells
-Activation of JAK1 and Tyk2
-Phosphorylates and activates STAT3
-NF-κB and MAPK pathways inhibited
-Reduces the production of pro-inflammatory cytokines
-Macrophage and dendritic cell regulation
-Modulation of T-cells, growth of regulatory T-cells
TGF-β
-Binding of TGF-β to TGF-β receptors I and II on targe
-SMAD2 and SMAD3 are activated
-SMAD4 complex formation
-Migration of SMAD complex to the nucleus
-Stimulates Tregs
-Reduction in pro-inflammatory cytokines
-Immune cell inhibition
Immune checkpoint inhibitors (ICIs) have revolutionized the treatment of non-small cell lung cancer (NSCLC); however, they are effective for only a handful of patients, hence appropriate biomarkers are required. Soluble biomarkers, such as sPD-1, sPD-L1, and cytokines, show potential for predicting and monitoring therapy results because of their accessibility and association with clinical reactions. Multiple studies establish that higher levels of sPD-1 are an adverse prognostic factor in NSCLC patients treated with immunotherapy. Levels of soluble sPDL-2 do appear to predict toxicity from immunotherapy but not survival. Elevated levels of sCTLA-4, sLAG-3, and CXCL12 appear to be adverse prognostic factors, but further studies are needed to confirm the findings on those markers. Furthermore, cytokines play an important role in tumor development and immunological regulation, supporting or inhibiting cancer progression. Pro-inflammatory cytokines (TNF-α, IL-6, and IL-1β) promote inflammation, angiogenesis, and tumor survival, whereas anti-inflammatory cytokines (IL-10, TGF-β) control immune responses. Cytokine levels may serve as biomarkers for predicting treatment results in NSCLC patients, with continuing research aimed at incorporating these biomarkers into customized cancer therapy. The focus for future studies needs to include clinical trials on large sample sizes, patients on combination therapy, other soluble components such as exosomes, longer follow-up periods and various ethnic groups to fully understand the role of these soluble mediators as immunological biomarkers in immunotherapy treated NSCLC patients.
| BRAF | v-Raf Murine Sarcoma Viral Oncogene Homolog B |
| CD274 | Cluster of Differentiation 274 |
| CD80 | Cluster of Differentiation 80 |
| CRP | C-Reactive Protein |
| CT | Computed Tomography |
| CTLA-4 | Cytotoxic T-Lymphocyte Antigen 4 |
| DC | Dendritic Cells |
| EGFR | Epidermal Growth Factor Receptor |
| EMT | Epithelial-Mesenchymal Transition |
| exoPD-L1 | exosomal PD-L1 |
| ICI | Immune Checkpoint Inhibitors |
| IFN-γ | Interferon-gamma γ |
| Ig-C | Immunoglobulin-Like Constant Domain |
| Ig-V | Immunoglobulin-Like Variable Domain |
| IL-1β | Interleukin-1 beta |
| IL-10 | Interleukin-10 |
| IL-12 | Interleukin-12 |
| IL-2 | Interleukin-2 |
| IL-6 | Interleukin-6 |
| IL-1RAcP | IL-1 Receptor Accessory Protein |
| IL-8 | Interleukin-8 |
| INOS | Inducible Nitric Oxide Synthase |
| irAEs | Immune-related Adverse Events |
| JAK | Janus Kinase |
| LAG-3 | Lymphocyte-activation Gene 3 |
| MAPK | Mitogen-Activated Protein Kinase |
| MDSCs | Myeloid Derived Suppressor Cells |
| NF-κB | Nuclear Factor Kappa B |
| NK | Natural Killer |
| NSCLC | Non-Small Cell Lung Cancer |
| ORR | Objective Response Rate |
| OS | Overall Survival |
| OX40 | Tumor Necrosis Factor Receptor Superfamily, Member 4 |
| PD-1 | Programmed Cell Death protein-1 |
| PD-L1 | Programmed Death Ligand-1 |
| PET | Positron Emission Tomography |
| PFS | Progression-Free Survival |
| SCLC | Small Cell Lung cancer |
| S-CTLA-4 | Stromal Cytotoxic T-Lymphocyte Antigen 4 |
| STAT-3 | Signal Transducer and Activator of Transcription 3 |
| sPD-1 | Soluble Programmed Death Protein-1 |
| sPD-L1 | Soluble Programmed Death Ligand-1 |
| sPD-L2 | Soluble Programmed Death Ligand-2 |
| TGF-β | Transforming Growth Factor-beta |
| TILs | Tumor-Infiltrating Lymphocytes |
| T regs | Regulatory T Cells |
| TCR | T Cell Receptor |
| T-CTLA-4 | Tumors’ Cytotoxic T-Lymphocyte Antigen 4 |
| Th1/Th2 | Helper T cells 1/2 |
| TNF-α | Tumor Necrosis Factor-α |
| Tyk2 | Tyrosine Kinase 2 |
| VEGF | Vascular Endothelial Growth Factor |
F.H., K.S. and A.R. conceptualized and wrote the manuscript. K.S. prepared the figure; F.H. prepared the tables. All authors have read and agreed to the published version of the manuscript.
The authors declare that they have no conflicts of interest.
The authors didn’t receive any external funding for the manuscript.
The Quilbolt paraphrasing tool was used in some parts to improve texts, and English editing ensuring that sentences were properly structured to enable better understanding.
[1] H. Sung, J. Ferlay, R.L. Siegel, M. Laversanne, I. Soerjomataram, A. Jemal, et al., "Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries" CA A Cancer J. Clin., vol. 71, pp. 209-249, 2021. [Crossref] [PubMed]
[2] R.L. Siegel, K.D. Miller, A. Jemal, "Cancer statistics, 2019" CA A Cancer J. Clin., vol. 69, pp. 7-34, 2019. [Crossref]
[3] J.R. Molina, P. Yang, S.D. Cassivi, S.E. Schild, A.A. Adjei, "Non- small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship" Symp. Solid Tumors, vol. 83, pp. 584-594, 2008. [Crossref]
[4] S. Hussain, I. Mubeen, N. Ullah, S.S.U.D. Shah, B.A. Khan, M. Zahoor, et al., "Modern diagnostic imaging technique applications and risk factors in the medical field: A review" BioMed Res. Int., vol. 2022, 2022. [Crossref]
[5] P.-Y. Xing, Y.-X. Zhu, L. Wang, Z.-G. Hui, S.-M. Liu, J.-S. Ren, et al., "What are the clinical symptoms and physical signs for non-small cell lung cancer before diagnosis is made? A nation-wide multicenter 10-year retrospective study in China" Cancer Med., vol. 8, pp. 4055-4069, 2019. [Crossref]
[6] A. Daga, A. Ansari, S. Patel, S. Mirza, R. Rawal, V. Umrania, "Current drugs and drug targets in non-small cell lung cancer: Limitations and opportunities" Asian Pac. J. Cancer Prev. APJCP, vol. 16, pp. 4147-4156, 2015. [Crossref]
[7] J.D. Twomey, B. Zhang, "Cancer Immunotherapy Update: FDA- Approved Checkpoint Inhibitors and Companion Diagnostics" AAPS J., vol. 23, p. 39, 2021. [Crossref] [PubMed]
[8] F.H.N. Hussain, A. Raza, "Immune checkpoint inhibitors in cancer immunotherapy," in Critical Developments in Cancer Immunotherapy, , Eds. New York, NY, USA: IGI Global, 2024, p. 34.
[9] S.L. Topalian, C.G. Drake, D.M. Pardoll, "Targeting the PD-1/B7- H1(PD-L1) pathway to activate anti-tumor immunity" Curr. Opin. Immunol., vol. 24, pp. 207-212, 2012. [Crossref]
[10] P. Sharma, S. Hu-Lieskovan, J.A. Wargo, A. Ribas, "Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy" Cell, vol. 168, pp. 707-723, 2017. [Crossref]
[11] A.B. Nixon, K.A. Schalper, I. Jacobs, S. Potluri, I.M. Wang, C. Fleener, "Peripheral immune-based biomarkers in cancer immunotherapy: Can we realize their predictive potential?" J. Immunother. Cancer, vol. 7, pp. 1-14, 2019. [Crossref]
[12] L. Pilla, C. Maccalli, "Immune Profiling of Cancer Patients Treated with Immunotherapy: Advances and Challenges" Biomedicines, vol. 6, 2018. [Crossref] [PubMed]
[13] A. Raza, M. Merhi, A. Relecom, Q. Fernandes, V. Inchakalody, A.R. Zar Gul, et al., "Evolving dynamic biomarkers for prediction of immune responses to checkpoint inhibitors in cancer," in Evolving Dynamic Biomarkers for Prediction of Immune Responses to Checkpoint Inhibitors in Cancer, , Eds. London, UK: IntechOpen, 2021, .
[14] A. Raza, R. Mohsen, A. Kanbour, A.R.Z. Gul, A. Philip, S. Vijayakumar, et al., "Serum immune mediators as novel predictors of response to anti-PD-1/PD-L1 therapy in non- small cell lung cancer patients with high tissue-PD-L1 expression" Front. Immunol., vol. 14, 2023. [Crossref] [PubMed]
[15] D. Gu, X. Ao, Y. Yang, Z. Chen, X. Xu, "Soluble immune checkpoints in cancer: Production, function and biological significance" J. Immunother. Cancer, vol. 6, p. 132, 2018. [Crossref]
[16] B.G. Neel, H. Gu, L. Pao, "The ‘Shp’ing news: SH2 domain- containing tyrosine phosphatases in cell signaling" Trends Biochem. Sci., vol. 28, pp. 284-293, 2003. [Crossref] [PubMed]
[17] M. Ahmadzadeh, L.A. Johnson, B. Heemskerk, J.R. Wunderlich, M.E. Dudley, D.E. White, et al., "Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired" Blood, vol. 114, pp. 1537-1544, 2009. [Crossref]
[18] A. Salmaninejad, V. Khoramshahi, A. Azani, E. Soltaninejad, S. Aslani, M.R. Zamani, et al., "PD-1 and cancer: Molecular mechanisms and polymorphisms" Immunogenetics, vol. 70, pp. 73-86, 2018. [Crossref]
[19] X. Zhu, J. Lang, "Soluble PD-1 and PD-L1: Predictive and prognostic significance in cancer" Oncotarget, vol. 8, pp. 97671-97682, 2017. [Crossref]
[20] M.Y. Song, S.H. Park, H.J. Nam, D.H. Choi, Y.C. Sung, "Enhancement of vaccine-induced primary and memory CD8(+) T-cell responses by soluble PD-1" J. Immunother. (Hagerstown Md. 1997), vol. 34, pp. 297-306, 2011. [Crossref]
[21] L. He, G. Zhang, Y. He, H. Zhu, H. Zhang, Z. Feng, "Blockade of B7-H1 with sPD-1 improves immunity against murine hepatocarcinoma" Anticancer. Res., vol. 25, pp. 3309-3313, 2005.
[22] H. Kuipers, F. Muskens, M. Willart, D. Hijdra, F.B. van Assema, A.J. Coyle, et al., "Contribution of the PD-1 ligands/PD-1 signaling pathway to dendritic cell-mediated CD4+ T cell activation" Eur. J. Immunol., vol. 36, pp. 2472-2482, 2006. [Crossref]
[23] D. Daassi, K.M. Mahoney, G.J. Freeman, "The importance of exosomal PD-L1 in tumor immune evasion" Nat. Rev. Immunol., vol. 20, pp. 209-215, 2020. [Crossref]
[24] H. Himuro, Y. Nakahara, Y. Igarashi, T. Kouro, N. Higashijima, N. Matsuo, et al., "Clinical roles of soluble PD-1 and PD-L1 in plasma of NSCLC patients treated with immune checkpoint inhibitors" Cancer Immunol. Immunother., vol. 72, pp. 2829-2840, 2023. [Crossref]
[25] M. Tiako Meyo, A. Jouinot, E. Giroux-Leprieur, E. Fabre, M. Wislez, M. Alifano, et al., "Predictive Value of Soluble PD-1, PD-L1, VEGFA, CD40 Ligand and CD44 for Nivolumab Therapy in Advanced Non-Small Cell Lung Cancer: A Case- Control Study" Cancers, vol. 12, 2020. [Crossref]
[26] I.G. Zizzari, A. Di Filippo, F. Scirocchi, F.R. Di Pietro, H. Rahimi, A. Ugolini, et al., "Soluble Immune Checkpoints, Gut Metabolites and Performance Status as Parameters of Response to Nivolumab Treatment in NSCLC Patients" J. Pers. Med., vol. 10, 2020. [Crossref] [PubMed]
[27] Y.L. Alfranca, M.E.O. Garcia, A.G. Rueda, P.Á. Ballesteros, D.R. Rodríguez, M.T. Velasco, "Blood biomarkers of response to immune checkpoint inhibitors in non-small cell lung cancer" J. Clin. Med., vol. 11, 2022. [Crossref]
[28] N.B. Hassounah, V.S. Malladi, Y. Huang, S.S. Freeman, E.M. Beauchamp, S. Koyama, et al., "Identification and characterization of an alternative cancer-derived PD-L1 splice variant" Cancer Immunol. Immunother. CII, vol. 68, pp. 407-420, 2019. [Crossref]
[29] B. Gong, K. Kiyotani, S. Sakata, S. Nagano, S. Kumehara, S. Baba, et al., "Secreted PD-L1 variants mediate resistance to PD-L1 blockade therapy in non-small cell lung cancer" J. Exp. Med., vol. 216, pp. 982-1000, 2019. [Crossref]
[30] B. Han, L. Dong, J. Zhou, Y. Yang, J. Guo, Q. Xuan, et al., "The clinical implication of soluble PD-L1 (sPD-L1) in patients with breast cancer and its biological function in regulating the function of T lymphocyte" Cancer Immunol. Immunother. CII, vol. 70, pp. 2893-2909, 2021. [Crossref]
[31] Y. Li, Y. Xiao, M. Su, R. Zhang, J. Ding, X. Hao, et al., "Role of soluble programmed death-1 (sPD-1) and sPD-ligand 1 in patients with cystic echinococcosis" Exp. Ther. Med., vol. 11, pp. 251-256, 2016. [Crossref] [PubMed]
[32] K.M. Mahoney, S.A. Shukla, N. Patsoukis, A. Chaudhri, E.P. Browne, A. Arazi, et al., "A secreted PD-L1 splice variant that covalently dimerizes and mediates immunosuppression" Cancer Immunol. Immunother. CII, vol. 68, pp. 421-432, 2019. [Crossref]
[33] S. Murakami, R. Shibaki, Y. Matsumoto, T. Yoshida, Y. Goto, S. Kanda, et al., "Association between serum level soluble programmed cell death ligand 1 and prognosis in patients with non-small cell lung cancer treated with anti-PD-1 antibody" Thorac. Cancer, vol. 11, pp. 3585-3595, 2020. [Crossref]
[34] Y. Okuma, H. Wakui, H. Utsumi, Y. Sagawa, Y. Hosomi, K. Kuwano, et al., "Soluble programmed cell death ligand 1 as a novel biomarker for nivolumab therapy for non-small-cell lung cancer" Clin. Lung Cancer, vol. 19, pp. 410-417.e1, 2018. [Crossref]
[35] I. Chmielewska, A. Grenda, P. Krawczyk, M. Frąk, B.K. Kamińska, W. Mitura, et al., "The influence of plasma sPD-L1 concentration on the effectiveness of immunotherapy in advanced NSCLC patients" Cancer Immunol. Immunother., vol. 72, pp. 4169-4177, 2023. [Crossref]
[36] A. Costantini, C. Julie, C. Dumenil, Z. Hélias-Rodzewicz, J. Tisserand, J. Dumoulin, et al., "Predictive role of plasmatic biomarkers in advanced non- small cell lung cancer treated by nivolumab" Oncoimmunology, vol. 7, p. e1452581, 2018. [Crossref]
[37] M. Bonomi, T. Ahmed, S. Addo, M. Kooshki, D. Palmieri, B.J. Levine, et al., "Circulating immune biomarkers as predictors of the response to pembrolizumab and weekly low dose carboplatin and paclitaxel in NSCLC and poor PS: An interim analysis" Oncol. Lett., vol. 17, pp. 1349-1356, 2019. [Crossref] [PubMed]
[38] S.A. Patel, D.E. Gerber, A. Deal, K. Douglas, C.V. Pecot, C. Lee, et al., "Consolidation with pembrolizumab and nab-paclitaxel after induction platinum-based chemotherapy for advanced non-small cell lung cancer" Front. Oncol., vol. 11, 2021. [Crossref] [PubMed]
[39] Y. Latchman, C.R. Wood, T. Chernova, D. Chaudhary, M. Borde, I. Chernova, et al., "PD-L2 is a second ligand for PD-1 and inhibits T cell activation" Nat. Immunol., vol. 2, pp. 261-268, 2001. [Crossref]
[40] A.H. Sharpe, E.J. Wherry, R. Ahmed, G.J. Freeman, "The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection" Nat. Immunol., vol. 8, pp. 239-245, 2007. [Crossref]
[41] L.T. Nguyen, S. Radhakrishnan, B. Ciric, K. Tamada, T. Shin, D.M. Pardoll, et al., "Cross-linking the B7 family molecule B7-DC directly activates immune functions of dendritic cells" J. Exp. Med., vol. 196, pp. 1393-1398, 2002. [Crossref]
[42] X. Cheng, V. Veverka, A. Radhakrishnan, L.C. Waters, F.W. Muskett, S.H. Morgan, et al., "Structure and interactions of the human programmed cell death 1 receptor" J. Biol. Chem., vol. 288, pp. 11771-11785, 2013. [Crossref]
[43] E. Lázár-Molnár, Q. Yan, E. Cao, U. Ramagopal, S.G. Nathenson, S.C. Almo, "Crystal structure of the complex between programmed death-1 (PD-1) and its ligand PD-L2" Proc. Natl. Acad. Sci. USA, vol. 105, pp. 10483-10488, 2008. [Crossref]
[44] E.A. Philips, A. Garcia-España, A.S. Tocheva, I.M. Ahearn, K.R. Adam, R. Pan, et al., "The structural features that distinguish PD-L2 from PD-L1 emerged in placental mammals" J. Biol. Chem., vol. 295, pp. 4372-4380, 2020. [Crossref]
[45] S. Tang, P.S. Kim, "A high-affinity human PD-1/PD-L2 complex informs avenues for small-molecule immune checkpoint drug discovery" Proc. Natl. Acad. Sci. USA, vol. 116, pp. 24500-24506, 2019. [Crossref]
[46] X.H. He, Y. Liu, L.H. Xu, Y.Y. Zeng, "Cloning and identification of two novel splice variants of human PD-L2" Acta Biochim. Et Biophys. Sin., vol. 36, pp. 284-289, 2004. [Crossref] [PubMed]
[47] J. Ancel, V. Dormoy, B. Nawrocki Raby, V. Dalstein, A. Durlach, M. Dewolf, et al., "Soluble biomarkers to predict clinical outcomes in non-small cell lung cancer treated by immune checkpoint inhibitors" Front. Immunol., vol. 14, 2023. [Crossref]
[48] P.T. Kennedy, E.L. Saulters, A.D. Duckworth, Y.J. Lim, J.F. Woolley, J.R. Slupsky, et al., "Soluble CTLA- 4 attenuates T cell activation and modulates anti-tumor immunity" Mol. Ther., vol. 32, pp. 457-468, 2023. [Crossref] [PubMed]
[49] F.J. Ward, L.N. Dahal, S.K. Wijesekera, S.K. Abdul-Jawad, T. Kaewarpai, H. Xu, et al., "The soluble isoform of CTLA-4 as a regulator of T-cell responses" Eur. J. Immunol., vol. 43, pp. 1274-1285, 2013. [Crossref]
[50] A.C. Tan, S.L. Cook, M. Khasraw, "Soluble immune-checkpoint factors: A potential immunotherapy biomarker" J. Clin. Investig., vol. 134, p. e179352, 2024. [Crossref]
[51] H. Hayashi, K. Chamoto, R. Hatae, T. Kurosaki, Y. Togashi, K. Fukuoka, et al., "Soluble immune checkpoint factors reflect exhaustion of antitumor immunity and response to PD-1 blockade" J. Clin. Investig., vol. 134, p. e168318, 2024. [Crossref]
[52] J. Liu, X. Tian, Y. Wang, X. Kang, W. Song, "Soluble cytotoxic T- lymphocyte-associated antigen 4 (sCTLA-4) as a potential biomarker for diagnosis and evaluation of the prognosis in Glioma" BMC Immunol., vol. 22, 2021. [Crossref]
[53] E. Pawlak, I.E. Kochanowska, I. Frydecka, M. Kiełbiński, S. Potoczek, M. Bilińska, "The soluble CTLA-4 receptor: A new marker in autoimmune diseases" Arch. Immunol. Et Ther. Exp., vol. 53, pp. 336-341, 2005.
[54] B. Kavanagh, S. O’Brien, D. Lee, Y. Hou, V. Weinberg, B. Rini, et al., "CTLA4 blockade expands FoxP3+ regulatory and activated effector CD4+ T cells in a dose-dependent fashion" Blood, vol. 112, pp. 1175-1183, 2008. [Crossref] [PubMed]
[55] X.H. Jia, L.Y. Geng, P.P. Jiang, H. Xu, K.J. Nan, Y. Yao, et al., "The biomarkers related to immune-related adverse events caused by immune checkpoint inhibitors" J. Exp. Clin. Cancer Res., vol. 39, p. 284, 2020. [Crossref]
[56] E.I. Buchbinder, A. Desai, "CTLA-4 and PD-1 pathways: Similarities, differences, and implications of their inhibition" Am. J. Clin. Oncol., vol. 39, pp. 98-106, 2016. [Crossref]
[57] R. Simone, G. Pesce, P. Antola, M. Rumbullaku, M. Bagnasco, N. Bizzaro, et al., "The soluble form of CTLA-4 from serum of patients with autoimmune diseases regulates T-cell responses" BioMed Res. Int., vol. 2014, 2014. [Crossref] [PubMed]
[58] E.E. Paulsen, T.K. Kilvaer, M. Rakaee, E. Richardsen, S.M. Hald, S. Andersen, et al., "CTLA-4 expression in the non-small cell lung cancer patient tumor microenvironment: Diverging prognostic impact in primary tumors and lymph node metastases" Cancer Immunol. Immunother., vol. 66, pp. 1449-1461, 2017. [Crossref] [PubMed]
[59] S. Gertel, A. Polachek, O. Elkayam, V. Furer, "Lymphocyte activation gene-3 (LAG-3) regulatory T cells: An evolving biomarker for treatment response in autoimmune diseases" Autoimmun. Rev., vol. 21, p. 103085, 2022. [Crossref]
[60] E. Ruffo, R.C. Wu, T.C. Bruno, C.J. Workman, D.A.A. Vignali, "Lymphocyte-activation gene 3 (LAG3): The next immune checkpoint receptor" Semin. Immunol., vol. 42, p. 101305, 2019. [Crossref]
[61] L.V. Mejía-Guarnizo, P.S. Monroy-Camacho, A.D. Turizo-Smith, J.A. Rodríguez-García, "The role of immune checkpoints in antitumor response: A potential antitumor immunotherapy" Front. Immunol., vol. 14, 2023. [Crossref]
[62] J. Ocarro, E. Blanco, M. Zuazo, H. Arasanz, A. Bocanegra, L. Fernández-Rubio, et al., "The role of immune checkpoints in antitumor response: A potential antitumor immunotherapy" Int. J. Mol. Sci., vol. 22, 2021. [Crossref]
[63] R.A. Mariuzza, S. Shahid, S.S. Karade, "The immune checkpoint receptor LAG3: Structure, function, and target for cancer immunotherapy" J. Biol. Chem., vol. 300, 2024. [Crossref] [PubMed]
[64] J. Gorgulho, C. Roderburg, F. Beier, C. Bokemeyer, T.H. Brümmendorf, S.H. Loosen, et al., "Soluble lymphocyte activation gene- 3 (sLAG3) and CD4/CD8 ratio dynamics as predictive biomarkers in patients undergoing immune checkpoint blockade for solid malignancies" Br. J. Cancer, vol. 130, pp. 1013-1022, 2024. [Crossref]
[65] L.P. Andrews, A.E. Marciscano, C.G. Drake, D.A. Vignali, "LAG3 (CD223) as a cancer immunotherapy target" Immunol. Rev., vol. 276, pp. 80-96, 2017. [Crossref]
[66] Y. He, Y. Wang, S. Zhao, C. Zhao, C. Zhou, F.R. Hirsch, "sLAG-3 in non-small-cell lung cancer patients’ serum" OncoTargets Ther., vol. 11, pp. 4781-4784, 2018. [Crossref]
[67] R. Kalluri, V.S. LeBleu, "The biology, function, and biomedical applications of exosomes" Science, vol. 367, p. eaau6977, 2020. [Crossref]
[68] T.L. Whiteside, "Exosomes and tumor-mediated immune suppression" J. Clin. Investig., vol. 126, pp. 1216-1223, 2016. [Crossref]
[69] B. Honrubia-Peris, J. Garde-Noguera, J. García-Sánchez, N. Piera-Molons, A. Llombart-Cussac, M.L. Fernández-Murga, "Soluble biomarkers with prognostic and predictive value in advanced non-small cell lung cancer treated with immunotherapy" Cancers, vol. 13, 2021. [Crossref] [PubMed]
[70] L. Ye, Z. Zhu, X. Chen, H. Zhang, J. Huang, S. Gu, et al., "The importance of exosomal PD-L1 in cancer progression and its potential as a therapeutic target" Cells, vol. 10, 2021. [Crossref]
[71] M. Del Re, R. Marconcini, G. Pasquini, E. Rofi, C. Vivaldi, F. Bloise, et al., "PD-L1 mRNA expression in plasma-derived exosomes is associated with response to anti-PD-1 antibodies in melanoma and NSCLC" Br. J. Cancer, vol. 118, pp. 820-824, 2018. [Crossref]
[72] S. Akbar, A. Raza, R. Mohsin, A. Kanbour, S. Qadri, A. Parray, et al., "Circulating exosomal immuno-oncological checkpoints and cytokines are potential biomarkers to monitor tumor response to anti-PD-1/PD-L1 therapy in non-small cell lung cancer patients" Front. Immunol., vol. 13, 2023. [Crossref]
[73] C. Li, C. Li, C. Zhi, Y. Gao, "Clinical significance of PD-L1 expression in serum-derived exosomes in NSCLC patients" J. Transl. Med., vol. 17, p. 355, 2019. [Crossref]
[74] K.C. Conlon, M.D. Miljkovic, T.A. Waldmann, "Cytokines in the treatment of cancer" J. Interferon Cytokine Res., vol. 39, pp. 6-21, 2019. [Crossref] [PubMed]
[75] N. Stanilov, T. Velikova, S. Stanilova, "Navigating the cytokine seas: Targeting cytokine signaling pathways in cancer therapy" Int. J. Mol. Sci., vol. 25, 2024. [Crossref] [PubMed]
[76] S.L. Topalian, "Targeting immune checkpoints in cancer therapy" JAMA, vol. 318, pp. 1647-1648, 2017. [Crossref]
[77] M. Yi, T. Li, M. Niu, H. Zhang, Y. Wu, K. Wu, et al., "Targeting cytokine and chemokine signaling pathways for cancer therapy" Signal Transduct. Target. Ther., vol. 9, p. 176, 2024. [Crossref] [PubMed]
[78] G. Tobelem, "VEGF: A key therapeutic target for the treatment of cancer—Insights into its role and pharmacological inhibition" Target. Oncol., vol. 2, pp. 153-164, 2007. [Crossref]
[79] L. Paz-Ares, T.M. Kim, D. Vicente, E. Felip, D.H. Lee, K.H. Lee, et al., "Bintrafusp Alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in second-line treatment of patients with NSCLC: Results from an expansion cohort of a phase 1 trial" J. Thorac. Oncol., vol. 15, pp. 1210-1222, 2020. [Crossref]
[80] S.A. Patel, M.B. Nilsson, X. Le, T. Cascone, R.K. Jain, J.V. Heymach, "Molecular mechanisms and future implications of VEGF/VEGFR in cancer therapy" Clin. Cancer Res., vol. 29, pp. 30-39, 2023. [Crossref]
[81] V. Ramundo, M.L. Palazzo, E. Aldieri, "TGF-β as predictive marker and pharmacological target in lung cancer approach" Cancers, vol. 15, 2023. [Crossref]
[82] M. Rašková, L. Lacina, Z. Kejík, A. Venhauerová, M. Skaličková, M. Kolář, et al., "The role of IL-6 in cancer cell invasiveness and metastasis—Overview and therapeutic opportunities" Cells, vol. 11, 2022. [Crossref]
[83] J. Chen, Y. Wei, W. Yang, Q. Huang, Y. Chen, K. Zeng, et al., "IL-6: The link between inflammation, immunity, and breast cancer" Front. Oncol., vol. 12, 2022. [Crossref] [PubMed]
[84] T. Tanaka, M. Narazaki, T. Kishimoto, "IL-6 in inflammation, immunity, and disease" Cold Spring Harb. Perspect. Biol., vol. 6, 2014. [Crossref]
[85] D. Johnson, R. O’Keefe, J. Grandis, "Targeting the IL-6/JAK/STAT3 signalling axis in cancer" Nat. Rev. Clin. Oncol., vol. 15, pp. 234-248, 2018. [Crossref]
[86] K. Ren, R. Torres, "Role of interleukin-1β during pain and inflammation" Brain Res. Rev., vol. 60, pp. 57-64, 2009. [Crossref] [PubMed]
[87] C. Rébé, F. Ghiringhelli, "Interleukin-1β and cancer" Cancers, vol. 12, 2020. [Crossref]
[88] G. Lopez-Castejon, D. Brough, "Understanding the mechanism of IL- 1β secretion" Cytokine Growth Factor Rev., vol. 22, pp. 189-195, 2011. [Crossref]
[89] E.B. Garon, J.C.-H. Yang, S.M. Dubinett, "The role of interleukin 1β in the pathogenesis of lung cancer" J. Thorac. Oncol. Rep., vol. 1, p. 100001, 2020. [Crossref]
[90] H. Li, M. Wu, X. Zhao, "Role of chemokine systems in cancer and inflammatory diseases" MedComm, vol. 3, p. e147, 2022. [Crossref]
[91] L.M. Campbell, P.J. Maxwell, D.J.J. Waugh, "Rationale and means to target pro-inflammatory interleukin-8 (CXCL8) signaling in cancer" Pharmaceuticals, vol. 6, 2013. [Crossref]
[92] K. Fousek, L.A. Horn, C. Palena, "Interleukin-8: A chemokine at the intersection of cancer plasticity, angiogenesis, and immune suppression" Pharmacol. Ther., vol. 219, p. 107692, 2021. [Crossref]
[93] M. Gonzalez-Aparicio, C. Alfaro, "Significance of the IL-8 pathway for immunotherapy" Hum. Vaccines Immunother., vol. 16, pp. 2312-2317, 2019. [Crossref]
[94] Y. Ge, M. Huang, Y.-m. Yao, "Biology of interleukin-17 and its pathophysiological significance in sepsis" Front. Immunol., vol. 11, 2020. [Crossref]
[95] C. Zenobia, G. Hajishengallis, "Basic biology and role of interleukin-17 in immunity and inflammation" Periodontology 2000, vol. 69, pp. 142-159, 2015. [Crossref] [PubMed]
[96] S. Li, R. Na, X. Li, Y. Zhang, T. Zheng, "Targeting interleukin-17 enhances tumor response to immune checkpoint inhibitors in colorectal cancer" Biochim. Et Biophys. Acta (BBA) Rev. Cancer, vol. 1877, 2022. [Crossref]
[97] K.H.G. Mills, "IL-17 and IL-17-producing cells in protection versus pathology" Nat. Rev. Immunol., vol. 23, pp. 38-54, 2023. [Crossref]
[98] L. Huangfu, R. Li, Y. Huang, S. Wang, "The IL-17 family in diseases: From bench to bedside" Signal Transduct. Target. Ther., vol. 8, p. 402, 2023. [Crossref]
[99] R.M. Onishi, S.L. Gaffen, "Interleukin-17 and its target genes: Mechanisms of interleukin-17 function in disease" Immunology, vol. 129, pp. 311-321, 2010. [Crossref]
[100] D. Briukhovetska, J. Dörr, S. Endres, P. Libby, C.A. Dinarello, S. Kobold, "Interleukins in cancer: From biology to therapy" Nat. Rev. Cancer, vol. 21, pp. 481-499, 2021. [Crossref]
[101] M. Oft, "IL-10: Master switch from tumor-promoting inflammation to antitumor immunity" Cancer Immunol. Res., vol. 2, pp. 194-199, 2014. [Crossref]
[102] V. Carlini, D.M. Noonan, E. Abdalalem, D. Goletti, C. Sansone, L. Calabrone, et al., "The multifaceted nature of IL-10: Regulation, role in immunological homeostasis and its relevance to cancer, COVID-19 and post- COVID conditions" Front. Immunol., vol. 14, 2023. [Crossref]
[103] S.S. Iyer, G. Cheng, "Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease" Crit. Rev. Immunol., vol. 32, pp. 23-63, 2012. [Crossref] [PubMed]
[104] B. Bierie, H.L. Moses, "Transforming growth factor beta (TGF-β) and inflammation in cancer" Cytokine Growth Factor Rev., vol. 21, pp. 49-59, 2010. [Crossref] [PubMed]
[105] A.B. Baba, B. Rah, G.R. Bhat, I. Mushtaq, S. Parveen, R. Hassan, et al., "Transforming growth factor-beta (TGF-β) signaling in cancer—A betrayal within" Front. Pharmacol., vol. 13, 2022. [Crossref]
[106] E. Batlle, J. Massagué, "Transforming growth factor-β signaling in immunity and cancer" Immunity, vol. 50, pp. 924-940, 2019. [Crossref]
[107] Z. Deng, T. Fan, C. Xiao, H. Tian, Y. Zheng, C. Li, et al., "TGF-β signaling in health, disease and therapeutics" Signal Transduct. Target. Ther., vol. 9, p. 61, 2024. [Crossref] [PubMed]
[108] D. Kauffmann-Guerrero, K. Kahnert, R. Kiefl, L. Sellmer, J. Walter, J. Behr, et al., "Systemic inflammation and pro-inflammatory cytokine profile predict response to checkpoint inhibitor treatment in NSCLC: A prospective study" Sci. Rep., vol. 11, 2021. [Crossref]
[109] H. Schindler, F. Lusky, L. Daniello, M. Elshiaty, L. Gaissmaier, K. Benesova, et al., "Serum cytokines predict efficacy and toxicity but are not useful for disease monitoring in lung cancer treated with PD-(L)1 inhibitors" Front. Oncol., vol. 12, 2022. [Crossref]
[110] T. Wei, J. Zhang, Y. Qin, Y. Wu, L. Zhu, L. Lu, et al., "Increased expression of immunosuppressive molecules on intratumoral and circulating regulatory T cells in non-small-cell lung cancer patients" Am. J. Cancer Res., vol. 5, pp. 2190-2201, 2015.
[111] Y. Shi, X. Liu, J. Du, D. Zhang, J. Liu, M. Chen, et al., "Circulating cytokines associated with clinical outcomes in advanced non-small cell lung cancer patients who received chemoimmunotherapy" Thorac. Cancer, vol. 13, pp. 219-227, 2021. [Crossref]
[112] A. Passaro, M. Al Bakir, E.G. Hamilton, I.I. Wistuba, C. Swanton, S. Peters, "Cancer biomarkers: Emerging trends and clinical implications for personalized treatment" Cell, vol. 187, pp. 1617-1635, 2024. [Crossref]
[113] T. Abdul-Rahman, S. Ghosh, S.M. Badar, A. Nazir, G.B. Bamigbade, N. Aji, et al., "The paradoxical role of cytokines and chemokines at the tumor microenvironment: A comprehensive review" Eur. J. Med. Res., vol. 29, p. 124, 2024. [Crossref] [PubMed]
We use cookies to improve your experience on our site. By continuing to use our site, you accept our use of cookies. Learn more