
APA Style
Dia Advani, Joaquin Villarreal Barragan, Gianina Statache, Nadir Kadri, Nupur Kohli. (2025). Upcycled Mesenchymal Stem Cells: Repurposing Biological Waste Towards Sustainable Regenerative Therapies. Cell Therapy & Engineering Connect, 1 (Article ID: 0003). https://doi.org/10.69709/CellEngC.2025.101060MLA Style
Dia Advani, Joaquin Villarreal Barragan, Gianina Statache, Nadir Kadri, Nupur Kohli. "Upcycled Mesenchymal Stem Cells: Repurposing Biological Waste Towards Sustainable Regenerative Therapies". Cell Therapy & Engineering Connect, vol. 1, 2025, Article ID: 0003, https://doi.org/10.69709/CellEngC.2025.101060.Chicago Style
Dia Advani, Joaquin Villarreal Barragan, Gianina Statache, Nadir Kadri, Nupur Kohli. 2025. "Upcycled Mesenchymal Stem Cells: Repurposing Biological Waste Towards Sustainable Regenerative Therapies." Cell Therapy & Engineering Connect 1 (2025): 0003. https://doi.org/10.69709/CellEngC.2025.101060.
 ACCESS
Review Article
ACCESS
Review Article
Volume 1, Article ID: 2024.0003

Dia Advani
dia.jotwani@dubaihealth.ae

Joaquin Villarreal Barragan
100065160@ku.ac.ae

Gianina Statache
gianina.s@adscc.ae

Nadir Kadri
nadir.kadri@ki.se

Nupur Kohli
nupur.kohli@ku.ac.ae
1 Department of Biomedical Engineering and Biotechnology, Khalifa University of Science and Technology, Abu Dhabi 127788, UAE
2 Centre for Applied and Translational Genomics, Mohammed Bin Rashid University of Medicine and Health Sciences, Dubai Healthcare City, Dubai, 505055, UAE
3 Abu Dhabi Stem Cell Centre, Abu Dhabi 127788, UAE
4 Department of Laboratory medicine, Karolinska Institute, 171 77 Solna, Sweden
5 Healthcare Engineering Innovation Group, Khalifa University of Science and Technology, Abu Dhabi 127788, UAE
* Author to whom correspondence should be addressed
Received: 21 Nov 2024 Accepted: 13 Feb 2025 Available Online: 14 Feb 2025 Published: 21 Mar 2025
Over the last two decades, the use of adult stem cells in therapy has gained significant momentum. However, stem cells are usually associated with high extraction, expansion and storage costs. This is delaying their approval into clinical practice. By repurposing medical waste tissues for stem cell harvesting, there is an opportunity to extract valuable therapeutic material without incurring additional costs associated with procuring raw materials or handling waste disposal. Harvesting stem cells from discarded tissues is a non-invasive, safe procedure that lowers healthcare costs associated with managing donor site complications. Given the dire need for stem cells in regenerative therapies, it is imperative that necessary advancements are made towards reducing the gap between the supply and the demand of such cells for therapy. The innovative concept of “Upcycled mesenchymal Stem Cells (USCs)” has been proposed to upcycle and repurpose adult mesenchymal stem cells from biowastes. Summary has been presented regarding the regenerative applications, current clinical status, potential benefits and limitations of USC-based therapies.

The end of the 19th century marked the beginning of the concept of stem cells with self-renewal properties. Among these, a population of adult stem cells called mesenchymal stem cells (MSCs) have proven to be the prominent cell choice for various regenerative therapies. Self-renewal, multilineage differentiation, immunomodulation and paracrine effects are some of the documented properties of MSCs that have sparked great enthusiasm to unveil the hidden potential of these cells in different therapies [1]. MSCs are known to be obtained from a diverse range of tissues such as bone marrow, adipose tissue, skin, peripheral blood, muscles, synovial membrane, dental pulp, urine, menstrual blood, and birth-associated tissues such as placenta and umbilical cord [2]. The most commonly exploited source of human MSCs is the bone marrow. However, the isolation of MSCs from bone marrow, as well as other sources such as skin, peripheral blood, and muscles, is a highly invasive procedure that is accompanied by patient discomfort and the risk of infection. Therefore, research efforts are directed toward examining the regenerative potential of MSCs from biological wastes and other sustainable sources. Upcycling refers to the process of repurposing or transforming discarded or unused materials. In regenerative medicine, the term upcycled has previously been used for umbilical cord stem cells, as the umbilical cord is considered a rich source of MSCs and other types of stem cells [3]. Likewise, in a recently published study, the cells derived from waste prenatal or postnatal tissues/products are recognised as waste-derived stem-like cells (WDS-IC) [4]. Following this, a new term has been proposed to describe sustainable or green sources of MSCs as “Upcycled MSCs or USCs” which entail MSCs isolated from either birth-associated discarded tissues or the biological waste material discharged by the human body. The literature has been examined for sustainable sources of MSCs, summarizing the biological characteristics of USCs, their advantages over conventional MSCs, regenerative applications, and clinical status. USCs demonstrate potential to transform discarded stem cells into valuable therapeutics. For the purpose of this study, the term "conventional MSCs" refers to traditional sources of MSCs, such as those outlined in Table 1.
2.1. Birth-Associated Spare Parts Amnion/chorion membrane: The fetal membrane consists of two main layers: the amnion, which is the outermost layer surrounding the fetus and amniotic fluid, and the chorion, the innermost layer in direct contact with the maternal decidua. The specific substances secreted by these membranes govern amniotic fluid homeostasis and the cellular physiology of the maternal tissue. Their unique structural organisation and rich proteomic profile mark them as a potential source of stem cells. The amnion is the source of amniotic epithelial cells (hAECs) and amniotic mesenchymal stem or stromal cells (hAMSCs). hAMSCs can be extracted from the inner mesodermal tissue of the amnion by mechanical or enzymatic removal of the outer amniotic epithelial layer. These cells behave like fibroblasts and express different mesenchymal markers. Significantly, hAMSCs demonstrate multilineage potential and can differentiate into adipocytes, chondrocytes, osteoblasts and myocytes, as well as ectodermal lineages [5]. Likewise, the chorion is the source of chorionic mesenchymal stem cells (hCMSCs) which exhibit fibroblast-like characteristics and can be differentiated into conventional mesodermal lineages, including osteogenic, chondrogenic and adipogenic lineages [6]. Amniotic fluid: Amniotic fluid (AF) serves as the source of exchange of nutrients and other chemicals from the mother to the fetus. Traditionally, AFMSCs can be collected from AF either by the process of amniocentesis or during cesarean deliveries [7]. Although the collection of AF by amniocentesis is an invasive process, a non-invasive way of isolation from the medical waste discarded during C-section deliveries can be explored. The fluid is collected aseptically by a syringe and processed to remove erythrocytes, yielding a heterogeneous mixture of cells. After two subsequent passages, the cells become more homogenous with fibroblast-like morphology [8]. AF-MSCs exhibit a high self-renewal capacity and retain a normal karyotype even after successive divisions. Notably, they share similar morphological characteristics with BM-MSCs. Their greater differentiation capabilities are due to the presence of the human embryonic stem cell markers, octamer-binding transcription factor-4 (Oct-4) and stage-specific embryonic antigen-4 (SSEA-4). AF-MSCs show significant pluripotency and can successfully be differentiated into adipogenic, chondrogenic, endothelial, hepatic, myogenic, neurogenic, and osteogenic lineages under requisite conditions [9]. Umbilical cord and cord blood: The umbilical cord (UC) is an extra-embryonic tissue that is traditionally discarded after birth. UC comprises two umbilical arteries, one umbilical vein and Wharton’s jelly (WJ), a mucoid connective tissue. Various parts of UC have been explored as a source of MSCs including WJ, cord lining, and cord blood, in which WJ has been most widely studied. Accordingly, UC serves as a source of Wharton’s jelly MSCs (WJ-MSCs), cord lining MSCs (CLMCs) and cord blood-derived MSCs (UCB-MSCs) [10]. Several methods of UC-MSC isolation have been described, including the tissue explant method, mechanical dissociation followed by enzymatic digestion, and enzymatic digestion of WJ, with or without blood vessels [11]. UC-MSCs or WJ-MSCs specifically exhibit the expression of various MSC markers and adhesion molecule markers, while demonstrating low or negligible expression of immune response-related antigens and hematopoietic stem cell-associated surface antigens [12]. Several in-vitro findings suggested that UC-MSCs are highly plastic and can be differentiated into osteoblasts, cartilage, endothelial cells, neurons, cardiomyocytes, hepatic, and pancreatic cells. Moreover, UC-MSCs can modulate the immune system by secretion of various immunomodulatory cytokines regulating the functioning of different immune cells [13]. Maternal decidua: the maternal uterine bed nurturing the developing embryo, called the decidua, is discarded along with the placenta after childbirth. This tissue is the source of two cell populations - decidua basalis MSC (DBMSCs) and decidua parietalis MSC (DPMSCs), both of them represent a sustainable source of MSCs. For stem cell isolation, the decidua is typically enzymatically digested using collagenase and DNase enzymes, followed by filtration and centrifugation to extract the cells [14]. Although little research has been done to examine the functional characteristics of decidua-derived MSCs (DMSCs), they are known to differentiate into all three germ layers. Furthermore, they can differentiate into adipocytes, osteocytes and chondrocytes, while also secreting a wide range of growth factors and bioactive molecules with diverse functions [15,16]. 2.2. Adult Biological Waste Material Extracted tooth: The tooth is a highly vascularised, mineralised soft tissue that can serve as an easily accessible noncontroversial source of dental MSCs (DMSCs). Several types of DMSCs have been identified from the human adult permanent tooth, the deciduous tooth, oral mucosas and the periodontal ligament [17]. The pulp of human exfoliated deciduous teeth from children serves as a great disposable tissue to isolate stem cells from human exfoliated deciduous teeth (SHEDs). These stem cells can be isolated by using two methods: first is the enzymatic digestion of minced pulp tissue with collagenase and dispase, and the other is the tissue explant method that allows cells to grow out from the minced pulp on culture dishes [18]. They are characterised as highly plastic DMSCs with high population doubling time and express several embryonic stem cell markers on their surfaces [19]. SHEDs are reported to be differentiated into adipogenic, chondrogenic, endothelial, myogenic, hepatic, neuro-glial, osteogenic, odontogenic and pancreatic lineages [20]. DMSCs exclusively express various cell proliferation and extracellular matrix-related genes and are useful for various regenerative applications. Adipose tissue remnants: Subcutaneous adipose tissue serves as a rich source of stem cells that can be retrieved from liposuction procedures. The lipoaspirate is considered a biomedical waste and thus qualifies as a sustainable and easily accessible source of MSCs [21]. Adipose tissue-derived MSCs/stromal cells (AT-MSCs or ASCs) can be harvested either from liquid fat after the liposuction procedure or from solid fat retrieved from abdominoplasty. In a non-enzymatic method, a heterogeneous mixture of mature adipocytes is collected from the stromal vascular fraction (SVF) harvested after liposuction or resection by mechanical means. Besides, the enzymatic process involves collagenase digestion and centrifugation [22]. AT-MSCs are multipotent cells with high proliferation capacities with multilineage differentiation and immunosuppressive potential [23]. In studies, AT-MSCs have demonstrated the classical trilineage differentiation as BM-MSCs. Menstrual blood: Menstrual blood is a unique, easily accessible and sustainable source of menstrual blood-derived MSCs (MenSCs). The endometrial regeneration stem cell hypothesis suggests that adult stem cells in the uterine endometrium drive the continuous regeneration of shed endometrial tissue during menstruation [24]. Isolation of endometrial MSCs (eMSCs) is an invasive surgical process, while MenSCs can be easily collected from the discharged menstrual blood. Studies have shown that about two to four-fold higher frequency (0.04% to 0.02%) of MenSCs can be achieved from menstrual blood as compared to BM-MSCs [25]. In 2007, Meng et al. achieved a breakthrough by identifying and isolating endometrial regenerative cells as a novel alternative source of stem cells from menstrual fluid. MenSCs are isolated from menstrual blood by conventional density gradient centrifugation or direct red blood cell lysis treatment [26]. These are adherent cells with fibroblast-like morphology and possess high proliferation capacity. Like BM-MSCs, they can differentiate into varied mesodermal lineages and show some superior characteristics of differentiation into cardiomyocytes, neural cells, epidermal-like cells and hepatocytes. In terms of growth profile, MenSCs have exhibited higher cellular proliferation and in vitro migration properties with a 3.5-fold increase in colony-forming units (CFUs) in vitro as compared to BM-MSCs [27]. Urine: Human urine serves as a convenient, economical and safe way of isolating cells with self-renewal and multi-differentiation potential. Urine-derived stem cells (UdSCs) can be easily harvested from a patient’s voided urine samples, providing an inexpensive and minimally invasive method. These isolated stem cells express surface markers similar to those of mesenchymal stem cells (MSCs), renal cells, and other pluripotent cells [28]. UdSCs can be extracted from the urine samples by conventional centrifugation method and are generally cultured in keratinocyte serum-free media [29]. It has been evident from several studies that UdSCs have multipotent differentiation potential and can differentiate into the established MSC lineages as well as endothelial cells, neuronal cells, urothelial cells, podocytes and smooth muscle cells [30]. In addition, they secrete various angiogenic and immunomodulatory growth factors that are desirable for various therapeutic applications. Periapical cyst/lesions: Human periapical cysts represent the most frequent oral cysts formed as an inflammatory reaction to endodontic infection. For the first time, Marelli et al. reported and characterised a new MSC population called as human periapical cysts-MSCs (hPCy-MSCs) from human periapical lesions, a biological waste material [31]. These cells are isolated from the cystic wall by mechanical disruption followed by enzymatic digestion (collagenase and dispase) and culturing in a suitable medium [32]. The newly isolated MSCs were believed to have trilineage differentiation potential. Moreover, hPCy-MSCs express several neuronal markers and can differentiate into neurogenic-like cells. Easy harvest, self-renewal and high proliferation capacities, multipotency, proangiogenic properties and immunomodulatory actions mark them as an attractive source of dental MSCs [33]. Skin: The human skin is the largest organ of human body and serves as a source of multipotent stem cells. Human foreskin obtained during the circumcision procedure in new born babies is considered as a discarded surgical waste. Some recent studies have shown that foreskin-derived MSCs (FSK-MSCs) have multipotent and pluripotent properties and displayed multilineage differentiation and immunomodulatory actions [34]. Likewise, the excised burned human skin, which is generally discarded in routine procedures, is a host for viable burn-derived MSCs (BD-MSCs). These cells have shown comparable biological properties, including, population doubling time and colony formation with reduced differentiation potential when compared to UC-MSCs [35]. Comparison of conventional versus upcycled MSCs. Isolation procedure is invasive and painful Isolation procedure is invasive and painful Isolation procedure is invasive and requires skin biopsies Isolation procedure is invasive and requires biopsies Lower chondrogenic and adipogenic potential Isolation process in invasive Limited clinical evidence Limited clinical evidence No standardized isolation protocols Genetic instability due to source and culture conditions Inferior osteogenic and chondrogenic potential Limited studies available Donor variability Lower yield Limited studies available Limited studies available Limited studies available Limited accessibility
Sources
Isolation
Proliferation Capacity
Differentiation
CapacityKey Strengths
Limitations
Conventional MSCs
Bone marrow
[36,37]BM aspirate
Mean doubling time is 40 h, senescence after passage seven
Adipogenic, chondrogenic, osteogenic
Synovium/
synovial fluid
[38]Synovium and synovial fluid
Can proliferate up to passage ten
Adipogenic, chondrogenic, osteogenic
Skin [39]
Human skin biopsies
Doubling time is 7–8 days
Adipogenic, myogenic, osteogenic
Muscle [40]
Skeletal muscle tissue
Doubling time is 40 h
Adipogenic, blood cells, chondrogenic, hepatogenic, myogenic, neurogenic, osteogenic
Peripheral blood [41]
Mononuclear lymphocytes
Doubling time is 95 h
Adipogenic, chondrogenic, endothelial, osteogenic, neurogenic
Upcycled MSCs
Amnion/
Chorion [9]Embryonic amnion/chorion membrane
Doubling time is 36 h
Adipogenic, chondrogenic, osteogenic
Amniotic fluid [9]
Embryonic amniotic fluid
Doubling time is 36 h
Adipogenic, osteogenic, neurogenic
Placenta [42]
Placental tissue
Doubling time is 36 h
Adipogenic, endothelial, neurogenic, osteogenic
Umbilical cord and cord blood [13]
Umbilical cord and cord blood
Mean doubling time is 30 h
Adipogenic, chondrogenic, endothelial-like cells, neuron-like cells, osteogenic
Adipose tissue remnants [21,43]
Waste tissue remnants from liposuction or abdominoplasty
Mean doubling time is 4 ± 1 h, faster proliferation than BM-MSCs
Adipogenic, chondrogenic, muscular, neurogenic, osteogenic
Extracted tooth [44]
Extracted tooth
Mean doubling time is 19 h
Adipogenic, chondrogenic, hepatogenic, myogenic, neuronal, odontogenic, osteogenic
Menstrual blood [45]
Blood from menstrual cycle
Mean doubling time is 18–36 h
Adipogenic, chondrogenic, chardiomyocyte, hepatocytes, myogenic, osteogenic
Urine [28,30]
Voided human urine
Mean doubling time is 20–29 h for fresh urine and 28–32 h for preserved urine
Beta-like cells, chondrogenic, endothelial, myogenic, neuronal, osteogenic, smooth muscle cells, uroepithelial
Periapical cyst [31]
Dental cyst/lesion
Mean doubling time is 19 h
Adipogenic, neuronal, osteogenic
Foreskin [46]
Excised foreskin
Mean doubling time is 20–30 h
Adipogenic, chondrogenic, osteogenic
As of 16 August 2024, a comprehensive evaluation of the ClinicalTrials.gov database revealed a total of 1674 trials registered for various types of mesenchymal stem cells (MSCs). For upcycled MSCs, specific search was carried out using terms such as “amniotic fluid mesenchymal stem/stromal cells”, “adipose tissue mesenchymal stem/stromal cells”, “umbilical cord/cord blood mesenchymal stem/stromal cells”, and “Wharton’s jelly mesenchymal stem/stromal cells,” among others. This search yielded information on the total number of trials, clinical phases, statuses, and cell types involved. Repeated outcomes were excluded, considering only unique identifiers. The analysis showed that the majority of trials were conducted on umbilical cord-derived MSCs (UC-MSCs) and adipose tissue-derived MSCs (AT-MSCs), with no trial data available for amniotic fluid-derived MSCs (AF-MSCs), chorionic MSCs (hCMSCs), or periapical cyst-derived MSCs (hPCy-MSCs) (Figure 1A). USCs from other sources, such as exfoliated teeth, periapical cysts, menstrual blood and urine, have recently gained attention, and their clinical application is still unexplored. Among all the registered trials, most focused on phase I and II studies (Figure 1B), reflecting the early stage of clinical validation for these therapies. This analysis suggests that the long-term efficacy of MSC-based therapies remains insufficiently established, hindering broader clinical translation. Regarding trial statuses, the majority were either recruiting or completed (Figure 1C). For most clinical trials, allogenic MSCs were used, except for AT-MSCs, which are commonly employed in autologous applications (Figure 1D). Advantages of allogenic MSCs, such as low immunogenicity, ease of availability, and donor selection flexibility, position them as favourable for clinical use. However, limited data prevents definitive conclusions about the therapeutic superiority of allogenic versus autologous sources. Despite the abundance of completed trials, few have published results, complicating the assessment of clinical efficacy. Prominent examples include the CATO trial in the U.S., which explores intravenous UC-MSCs for heart failure, with expectations of significant outcomes [47]. The results from clinical trial NCT04355728, which assessed the efficacy of UC-MSC treatment for acute respiratory distress syndrome in COVID-19 patients, revealed positive outcomes with improved patient survival. The study demonstrated a significant reduction in inflammatory cytokines or “cytokine storm” in COVID-19 [36]. The safety and efficacy of UC-MSCs based therapies in a clinical trial (NCT03102879) for regenerative endodontic procedures (REPs) is another promising example of MSC clinical potential. In a 12-month follow-up study, patients were evaluated and shown positive pulp response and improved clinical efficacy with no adverse events [36]. The results from another clinical trial study (NCT03691909) illustrated the positive effects of autologous AD-MSC infusion in rheumatoid arthritis (RA) patients. AD-MSC treatment resulted in reduced C-reactive protein (CRP) levels in patients with significant improvement in joint symptoms, with no long-term adverse events reported [48]. The therapeutic benefits of AD-MSCs have been evaluated in another clinical trial (NCT03060551) in systemic sclerosis patients. After a 20-week follow-up period, patients with nebulised AD-MSC have shown improvement in hand edema, active ulcers and skin fibrosis with no adverse events [49]. One clinical study (NCT01385644) implemented PL-MSCs in pulmonary idiopathic fibrosis patients in a phase 1b trial. The published results revealed that the use of MSC-based cell therapy improved lung function in moderately fibrotic lung disease with a short-term safety profile [50]. In essence, although a minority of trials with upcycled MSCs have published results, the available data certainly provide an encouraging direction for further clinical investigation. A brief overview of some clinical trials for upcycled MSCs completed in 2023 is provided in Table 2. Examples of clinical trials with upcycled MSC completed in 2023. (Data retrieved from Clinicaltrials.gov).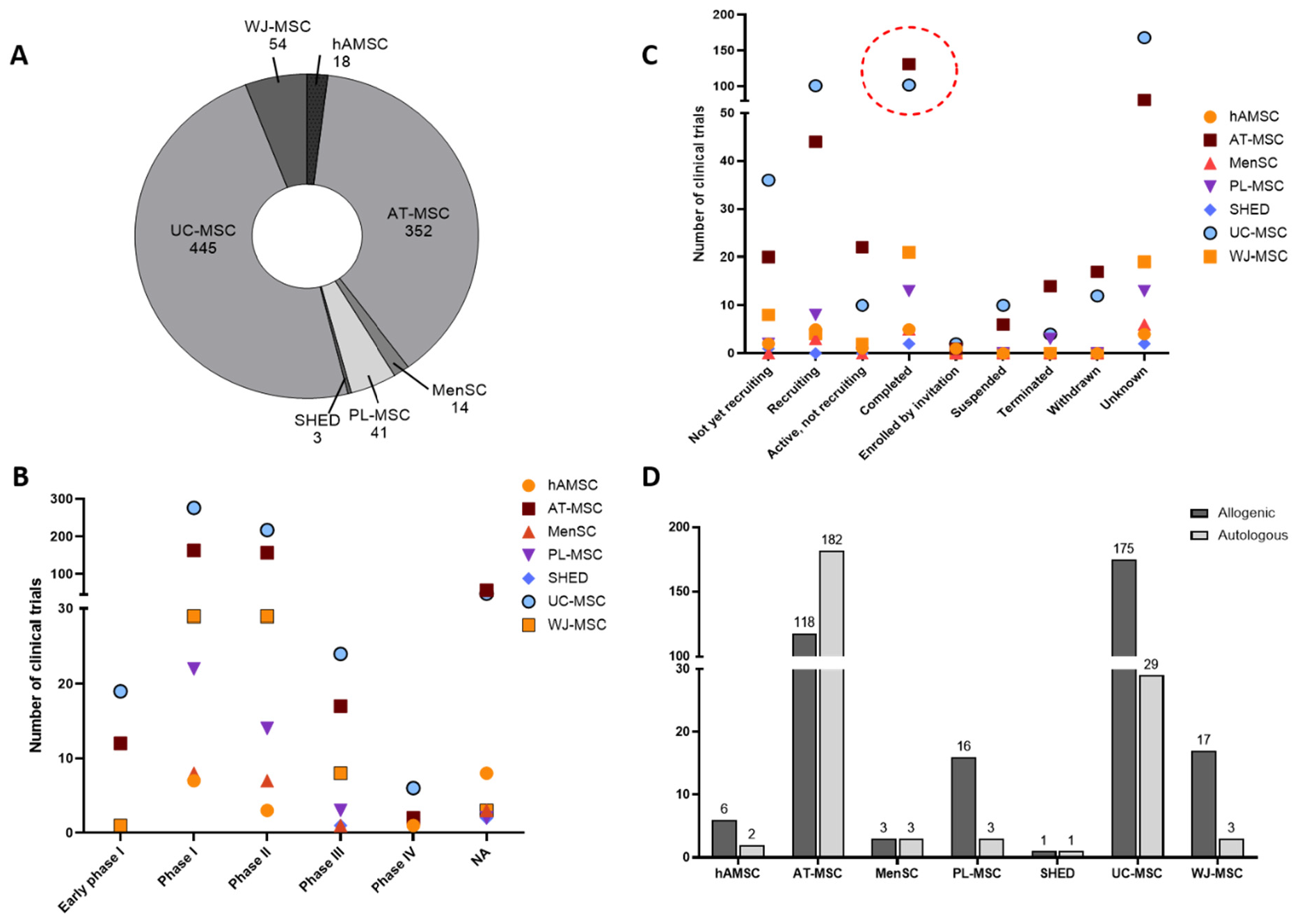
NCT Number
Study Phase
Study Design
Intervention
Cell Type
Condition
Completion Date
Summary
NCT05703308
III
Non-randomised study with 180 participants
Menstrual Blood Derived-Mesenchymal Stromal Cells
Autologous
Poor Ovarian Response/Female infertility
January, 2023
MenSC treatment has shown improvement in pregnancy outcomes in women after 2 month follow-up period with no reported side effects
NCT05777213
I
Open-label interventional study with 27 participants
Conditioned Medium Wharton’s Jelly-derived mesenchymal stem cells (CM-WJMSCs)
Allogenic
Ulcers
February, 2023
Study completed, results not available
NCT05279157
II
Randomised, parallel study with 15 participants
Human adipose-derived MSCs
Autologous
Corneal disease
February, 2023
Study completed, results not available
NCT04928287
II
Randomised, double-blind, single-centre study with 24 participants
Human adipose-derived MSCs
Autologous
Parkinson’s disease
February, 2023
Study completed, results not available
NCT03943576
I/II
Randomised, interventional study with 25 participants
Human adipose-derived MSCs (GXCPC1)
Allogenic
Knee osteoarthritis
March, 2023
Improved pain and knee function with no reported adverse event after one year of follow-up
NCT04325594
II
Non-randomised, open-label, interventional study with 30 participants
Human umbilical cord-derived MSCs
Allogenic
Chronic heart failure
April, 2023
Study completed, results not available
NCT03308006
II
Open-label, interventional study with 18 participants
Human adipose-derived MSCs
Allogenic
Knee osteoarthritis
April, 2023
Study completed, results not available
NCT03254758
I/II
Open-label, interventional study with 21 participants
Human adipose-derived MSCs
Allogenic
Liver cirrhosis
April, 2023
Study completed, results not available
NCT03183934
I/II
Open-label, observational study
Human adipose-derived MSCs ALLO-ASC-DFU
Allogenic
Dystrophic Epidermolysis Bullosa
April, 2023
Study completed, results not available
NCT04992832
I/II
Randomised, placebo, double-blind, interventional study with 0 participants
Human umbilical cord MSCs-derived secretome (PRIME-HFrEF)
Allogenic
Heart failure
April, 2023
Study completed, results not available
NCT04040348
I
Open-label, interventional study with 6 participants
Human umbilical cord MSCs
Allogenic
Alzheimer’s disease
April, 2023
Study completed, results not available
NCT04530071
I/II
Randomised, double-blind, placebo study with 36 participants
Human umbilical cord-derived MSCs (CordSTEM-DD)
Allogenic
Chronic low back pain
April, 2023
Study completed, results not available
NCT04040348
I
Open label, single group assignment with 6 participants
Human umbilical cord-derived MSCs
Allogenic
Alzheimer’s disease
April, 2023
Study completed, results not available
NCT05579665
I/II
Randomised, open-label, interventional study with 45 participants
Human umbilical cord MSCs-derived secretome
Allogenic
Knee osteoarthritis
May, 2023
Clinical improvement and biomarker changes in patients with mild to moderate disease with no side effects
NCT04738981
III
Randomised, open-label, interventional study with 130 participants
Human umbilical cord MSCs
Allogenic
Graft versus host disease
May, 2023
Better response after MSC therapy with no toxicity and adverse effects
NCT04208646
II
Multicentre, randomised, double blind study with 106 participants
Human adipose-derived MSCs (AlloJoin®)
Allogenic
Knee Osteoarthritis
July, 2023
Study completed, results not available
The significant biological properties of USCs make them an ideal source for regenerative applications, including the repair and regeneration of damaged tissues. The most recent applications of USCs focus on treating bone and cartilage defects, muscle degeneration, dental problems, liver failure, neuronal degeneration, and dental defects. Over the last decade, AD-MSCs have been extensively explored for tissue repair, wound healing and organ regeneration. AD-MSC cells are primarily used in autologous transfers for facial rejuvenation, cosmetic surgeries and reversal of skin necrosis [51]. Some studies have shown the potential of bioactive substances secreted by AD-MSCs in bone tissue engineering including bone repair and regeneration [52]. Likewise, USCs from dental sources have been documented to show better osteogenic potential promoting bone regeneration and reducing inflammation [53]. It has been demonstrated that MenSCs also contribute to wound healing and blood vessel formation by secreting some cytokine signals promoting cutaneous regeneration [54]. Beyond tissue and bone regeneration, a recent study reported on the applications of USCs from various sources, including UC-MSC, WJ-MSC, and AMSC, in cell-based and biomaterial-based neural regeneration [4]. After BM-MSC, AD-MSC and UC-MSC are the most extensively studied MSCs for treating nervous system inflammation, traumatic brain injuries and neuronal regeneration [55,56]. Studies have also shown that DMSCs have remarkable neurogenic potential due to the secretion of different neurotrophic factors and specifically, SHEDs are known for their neuroprotective activity [57,58]. Moreover, several preclinical and clinical studies revealed that the immunomodulatory properties of MSCs are beneficial for promoting liver regeneration. Similar to conventional BM-MSCs, AD-MSCs [59], UC-MSCs [60] and PL-MSCs [61] have shown differentiation into hepatocyte-like functional cells in 2D and 3D cultures. For dental applications, dental tissue-derived MSCs have widely been explored for periodontal tissue regeneration, dental pulp regeneration, tooth reconstruction and other dental tissue engineering applications. Besides, other USCs like AD-MSCs and UC-MSCs have also shown success in periodontitis and other dental diseases. Emerging studies continue to uncover novel applications for USCs in regenerative medicine. Their diverse biological properties, including immunomodulation and differentiation potential, underscore their versatility. Future research should prioritize long-term clinical studies to validate efficacy and explore underutilized sources, such as MenSCs and SHEDs, for broader clinical use.
Considering the limitations of conventional MSC sources, interest in biological waste products has recently been increased. USCs have comparable proliferative, migratory, immunomodulatory and anti-inflammatory properties and can be isolated by non-invasive procedures. Moreover, studies have shown that isolated MSCs from fat tissues during liposuction procedure can generate an entire network of blood vessels thus supporting tissue regeneration [62]. Despite having biological and medical benefits, USCs have additional advantages based on ethical and regulatory background. USC-based therapies are sustainable and help to reduce the burden of huge biomedical waste generated in hospitals and clinics. With each technological advancement, USC-based therapies have certain limitations and challenges. Cautions need to be considered from a safety perspective during the development of USC products. For instance, hemocompatibility, route of delivery of USC products, and potential adverse events after infusion of large cell doses should be considered. The in vivo phenotype of USC is still poorly defined in terms of hemocompatibility. Among USCs proposed, molecular profiling of PL-MSCs has revealed that these cells express higher levels of extravascular procoagulant factors compared to the prototypic hemocompatibility. It is proposed to perform careful hemocompatibility screening before clinical application, particularly when USCs are used systemically. Indeed, different USCs can secrete high levels of tissue factors (as shown for PL-MSCs) which might trigger coagulations. MSCs also express receptors for complement activation products that can trigger activation of the innate immune response [63]. Additional factors to be considered during studies of USCs would include effects of culture media/culture expansion, freeze-thawing, and the reconstitution buffer which might affect their phenotypes and secretion of tissues factors. Similar to the other so called conventional MSCs, it is also proposed to address USCs dosing, fitness, potency assays and careful investigation of the potential mechanism of action (might be indication dependent). Moreover, it is crucial to consider biosafety measures while isolating MSCs from biological waste material. Maintaining quality assurance and aseptic conditions, collecting in sterile containers, developing assays for testing potential contaminants during and after isolation, properly disposing of unused material, and maintaining detailed documentation are some of the essential biosafety measures to be considered. It is recommended to obtain a consent from the donors before using their biological waste for any research purpose. Additionally, complying with patient information and data privacy are critical measures to be considered to follow ethical and regulatory standards.
Repurposing of discarded tissues serves an alternate way to procure MSCs with an opportunity to overcome the additional financial burdens and reduce carbon foot print by minimising medical waste disposal. Furthermore, harvesting MSCs from discarded tissues typically involves a non-invasive procedure, in contrast to invasive approaches such as bone marrow aspiration or tissue biopsies. These surgical methods often involve huge expenditures due to anaesthesia, operating room utilisation, and postoperative care. Moreover, traditional harvesting procedures can lead to donor site morbidity, encompassing various complications such as discomfort, pain, or even infection. In addition, researchers face limitations in the quantity of source material available for cell expansion and various cell culture assays. The utilisation of discarded medical wastes as a source of MSC might circumvent the abovementioned issues and offer an ethically sound alternative for the integration of stem cell-based therapies. In essence, repurposing discarded tissues for stem cell harvesting provides a cost-effective alternative, overcoming the financial challenges of conventional stem cell extraction methods, and facilitating the potential integration of stem cell therapies into clinical applications. For specific diseases where patients are quite sick, have contraindications for bone marrow or adipose tissue extraction, or can’t wait for the manufacturing process, the availability of the shelf cells can make the clinical journey easier. However, the clinical success of USCs is challenged by factors such as variability and viability of isolated cells, inconsistent standardization and characterization protocols, hemocompatibility issues, reproducibility concerns, the impact of different delivery methods, and lack of extensive preclinical validations. To raise awareness within the scientific and regulatory communities, it is essential to investigate the hidden potential of various sources of USCs in experimental conditions and to translate and validate their efficacy in clinical settings.
| AF | Amniotic Fluid |
| AT-MSC | Adipose Tissue-Derived MSCs |
| BD-MSC | Burn-Derived MSC |
| CLMC | Cord Lining MSC |
| CRP | C-reactive protein |
| hAMSCs | Amniotic Mesenchymal Stem Cells |
| hPCy-MSC | Human Periapical Cysts-MSCs |
| DBMSC | Decidua Basalis MSC |
| DMSC | Dental-Tissue Derived MSC |
| DPMSC | Decidua Parietalis |
| FSK-MSC | Foreskin-Derived MSC |
| hAECs | Amniotic Epithelial Cells |
| MSC | Mesenchymal Stem Cells |
| MenSC | Menstrual Blood-Derived MSCs |
| Oct-4 | Octamer-Binding Transcription Factor-4 |
| REP | Regenerative Endodontic Procedures |
| SHED | Human Exfoliated Deciduous Teeth |
| SSEA-4 | Stage-Specific Embryonic Antigen-4 |
| SVF | Stromal Vascular Fraction |
| UDSC | Urine-Derived Stem Cells |
| USC | Upcycled Mesenchymal Stem Cells |
| UCB-MSC | Umbilical Cord Blood-Derived MSCs |
| UC | Umbilical cord |
| WJ | Wharton’s Jelly |
Conceptualization, methodology, software: D.A., J.K.B. and N.K. (Nupur Kohli); Validation, formal analysis, funding acquisition: D.A., J.K.B. and N.K. (Nupur Kohli); Investigation, resources, data curation, writing—original draft preparation, writing—review and editing, visualization, supervision, project administration: D.A., J.K.B, G.S., N.K. (Nadir Kadri) and N.K. (Nupur Kohli).
All figures included are original and not reproduced from any published source. The graphical abstract is created with BioRender.com.
The authors have no conflict of interest to declare.
This work was supported by Khalifa University faculty start-up grant (FSU-2022-023-8474000443).
The authors would like to acknowledge Khalifa University for their support during this study.
[1] M.F. Pittenger, D.E. Discher, B.M. Péault, D.G. Phinney, J.M. Hare, A.I. Caplan, "Mesenchymal stem cell perspective: Cell biology to clinical progress" NPJ Regen. Med., vol. 4, p. 22, 2019. [Crossref] [PubMed]
[2] R. Hass, C. Kasper, S. Böhm, R. Jacobs, "Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC" Cell Commun. Signal., vol. 9, p. 12, 2011. [Crossref]
[3] A. Moreira, Y. Alayli, S. Balgi, C. Winter, S. Kahlenberg, S. Mustafa, et al., "Upcycling Umbilical Cords: Bridging Regenerative Medicine with Neonatology" J. Matern. Fetal. Neonatal Med., vol. 32, p. 1378, 2017. [Crossref]
[4] Z. Eivazi Zadeh, S. Nour, S. Kianersi, F. Jonidi Shariatzadeh, R.J. Williams, D.R. Nisbet, et al., "Mining human clinical waste as a rich source of stem cells for neural regeneration" iScience, vol. 27, p. 110307, 2024. [Crossref]
[5] P.S. in ‘t Anker, S.A. Scherjon, C.K. der Keur, G.M.J.S. de Groot-Swings, F.H.J. Claas, W.E. Fibbe, et al., "Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta" Stem Cells, vol. 22, pp. 1338-1345, 2004. [Crossref] [PubMed]
[6] M. Soncini, E. Vertua, L. Gibelli, F. Zorzi, M. Denegri, A. Albertini, et al., "Isolation and characterization of mesenchymal cells from human fetal membranes" J. Tissue Eng. Regen. Med., vol. 1, pp. 296-305, 2007. [Crossref] [PubMed]
[7] J. Zhou, D. Wang, T. Liang, Q. Guo, G. Zhang, "Amniotic fluid-derived mesenchymal stem cells: Characteristics and therapeutic applications" Arch. Gynecol. Obstet., vol. 290, pp. 223-231, 2014. [Crossref]
[8] L.S. Spitzhorn, M.S. Rahman, L. Schwindt, H.T. Ho, W. Wruck, M. Bohndorf, et al., "Isolation and Molecular Characterization of Amniotic Fluid-Derived Mesenchymal Stem Cells Obtained from Caesarean Sections" Stem Cells Int., vol. 2017, p. 5932706, 2017. [Crossref]
[9] S.V. Murphy, A. Atala, "Amniotic fluid and placental membranes: Unexpected sources of highly multipotent cells" Semin. Reprod. Med., vol. 31, pp. 62-68, 2013. [Crossref]
[10] M. Das, A.J. Sloan, "Stem cell sources from human biological waste material: A role for the umbilical cord and dental pulp stem cells for regenerative medicine" Hum. Cell, vol. 36, pp. 1312-1325, 2023. [Crossref]
[11] J.R. Smith, K. Pfeifer, F. Petry, N. Powell, J. Delzeit, M.L. Weiss, "Standardizing Umbilical Cord Mesenchymal Stromal Cells for Translation to Clinical Use: Selection of GMP-Compliant Medium and a Simplified Isolation Method" Stem Cells Int., vol. 2016, p. 6810980, 2016. [Crossref] [PubMed]
[12] Y. Shang, H. Guan, F. Zhou, "Biological Characteristics of Umbilical Cord Mesenchymal Stem Cells and Its Therapeutic Potential for Hematological Disorders" Front. cell Dev. Biol., vol. 9, 2021. [Crossref] [PubMed]
[13] T.T. Sibov, P. Severino, L.C. Marti, L.F. Pavon, D.M. Oliveira, P.R. Tobo, et al., "Mesenchymal stem cells from umbilical cord blood: Parameters for isolation, characterization and adipogenic differentiation" Cytotechnology, vol. 64, pp. 511-521, 2012. [Crossref]
[14] E. Semenova, Z.R. Mrowiec, E.K. Machaj, M. Murzyn, K. Borg, D. Boruczkowski, et al., "Isolation and Characteristics of Mesenchymal Stromal Cells from Different Parts of Placenta" J. Stem Cell Res. Ther., vol. 7, p. 2, 2017. [Crossref]
[15] Y.-C. Huang, Z.-M. Yang, X.-H. Chen, M.-Y. Tan, J. Wang, X.-Q. Li, et al., "Isolation of mesenchymal stem cells from human placental decidua basalis and resistance to hypoxia and serum deprivation" Stem Cell Rev. Rep., vol. 5, pp. 247-255, 2009. [Crossref]
[16] F.M. Abomaray, M.A. Al Jumah, K.O. Alsaad, D. Jawdat, A. Al Khaldi, A.S. AlAskar, et al., "Phenotypic and Functional Characterization of Mesenchymal Stem/Multipotent Stromal Cells from Decidua Basalis of Human Term Placenta" Stem Cells Int., vol. 2016, p. 5184601, 2016. [Crossref]
[17] P.T. Sharpe, "Dental mesenchymal stem cells" Development, vol. 143, pp. 2273-2280, 2016. [Crossref]
[18] W. Sukarawan, T. Osathanon, W. Sukarawan, T. Osathanon, "Stem Cells from Human Exfoliated Deciduous Teeth: Biology and Therapeutic Potential," in Mesenchymal Stem Cells—Isolation, Characterization and Applications, , Eds. Rijeka, Croatia: IntechOpen, 2017, .
[19] S.-M. Lee, Q. Zhang, A.D. Le, "Dental Stem Cells: Sources and Potential Applications" Curr. Oral Health reports, vol. 1, pp. 34-42, 2014. [Crossref]
[20] R. Guo, J. Yu, "Multipotency and Immunomodulatory Benefits of Stem Cells from Human Exfoliated Deciduous Teeth" Front. Dent. Med., vol. 3, 2022. [Crossref]
[21] S. Schneider, M. Unger, M. Van Griensven, E.R. Balmayor, "Adipose-derived mesenchymal stem cells from liposuction and resected fat are feasible sources for regenerative medicine" Eur. J. Med. Res., vol. 22, pp. 1-11, 2017. [Crossref]
[22] P. Goulas, M. Karakwta, A. Zatagias, M. Bakoutsi, A. Zevgaridis, A. Ioannidis, et al., "A Simple and Effective Mechanical Method for Adipose-Derived Stromal Vascular Fraction Isolation" Cureus, vol. 16, p. e57137, 2024. [Crossref] [PubMed]
[23] P.C. Baer, H. Geiger, "Adipose-derived mesenchymal stromal/stem cells: Tissue localization, characterization, and heterogeneity" Stem Cells Int., vol. 2012, p. 812693, 2012. [Crossref] [PubMed]
[24] C.E. Gargett, R.W.S. Chan, K.E. Schwab, "Endometrial stem cells" Curr. Opin. Obstet. Gynecol., vol. 19, pp. 377-383, 2007. [Crossref]
[25] X. Meng, T.E. Ichim, J. Zhong, A. Rogers, Z. Yin, J. Jackson, et al., "Endometrial regenerative cells: A novel stem cell population" J. Transl. Med., vol. 5, p. 57, 2007. [Crossref] [PubMed]
[26] Y. Sun, Y. Ren, F. Yang, Y. He, S. Liang, L. Guan, et al., "High-yield isolation of menstrual blood-derived endometrial stem cells by direct red blood cell lysis treatment" Biol. Open, vol. 8, 2019. [Crossref]
[27] F. Alcayaga-Miranda, J. Cuenca, P. Luz-Crawford, C. Aguila-Díaz, A. Fernandez, F.E. Figueroa, et al., "Characterization of menstrual stem cells: Angiogenic effect, migration and hematopoietic stem cell support in comparison with bone marrow mesenchymal stem cells" Stem Cell Res. Ther., vol. 6, pp. 32-35, 2015. [Crossref]
[28] N. Pavathuparambil Abdul Manaph, M. Al-Hawwas, L. Bobrovskaya, P.T. Coates, X.-F. Zhou, "Urine-derived cells for human cell therapy" Stem Cell Res. Ther., vol. 9, pp. 1-12, 2018. [Crossref]
[29] Q. Zhou, Y. Cheng, F. Sun, J. Shen, M.I. Nasser, P. Zhu, et al., "A Comprehensive Review of the Therapeutic Value of Urine-Derived Stem Cells" Front. Genet., vol. 12, 2022. [Crossref]
[30] P. Burdeyron, S. Giraud, T. Hauet, C. Steichen, "Urine-derived stem/progenitor cells: A focus on their characterization and potential" World J. Stem Cells, vol. 12, pp. 1080-1096, 2020. [Crossref]
[31] A. Roi, C. Roi, M.L. Negruțiu, L.C. Rusu, M. Riviș, "Mesenchymal Stem Cells Derived from Human Periapical Cysts and Their Implications in Regenerative Medicine" Biomedicines, vol. 11, 2023. [Crossref]
[32] M. Tatullo, B. Codispoti, A. Pacifici, F. Palmieri, M. Marrelli, L. Pacifici, et al., "Potential Use of Human Periapical Cyst-Mesenchymal Stem Cells (hPCy-MSCs) as a Novel Stem Cell Source for Regenerative Medicine Applications" Front. Cell Dev. Biol., vol. 5, 2017. [Crossref] [PubMed]
[33] C. Brizuela, G. Meza, D. Urrejola, M.A. Quezada, G. Concha, V. Ramírez, et al., "Cell-Based Regenerative Endodontics for Treatment of Periapical Lesions: A Randomized, Controlled Phase I/II Clinical Trial" J. Dent. Res., vol. 99, pp. 523-529, 2020. [Crossref] [PubMed]
[34] Y. Xin, P. Xu, X. Wang, Y. Chen, Z. Zhang, Y. Zhang, "Human foreskin-derived dermal stem/progenitor cell-conditioned medium combined with hyaluronic acid promotes extracellular matrix regeneration in diabetic wounds" Stem Cell Res. Ther., vol. 12, pp. 1-18, 2021. [Crossref]
[35] R. Dolp, G. Eylert, C. Auger, A. Aijaz, Y.A. Chen, S. Amini-Nik, et al., "Biological characteristics of stem cells derived from burned skin—A comparative study with umbilical cord stem cells" Stem Cell Res. Ther., vol. 12, pp. 1-14, 2021. [Crossref]
[36] D. Baksh, R. Yao, R.S. Tuan, "Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow" Stem Cells, vol. 25, pp. 1384-1392, 2007. [Crossref] [PubMed]
[37] F.A.M. Abo-Aziza, A.K.A. Zaki, A.M.A. El-Maaty, "Bone marrow-derived mesenchymal stem cell (BM-MSC): A tool of cell therapy in hydatid experimentally infected rats" Cell Regen., vol. 8, pp. 58-71, 2019. [Crossref]
[38] M. Jeyaraman, S. Muthu, N. Jeyaraman, R. Ranjan, S.K. Jha, P. Mishra, "Synovium Derived Mesenchymal Stromal Cells (Sy-MSCs): A Promising Therapeutic Paradigm in the Management of Knee Osteoarthritis" Indian J. Orthop., vol. 56, pp. 1-15, 2022. [Crossref]
[39] K. Sellheyer, D. Krahl, "Skin mesenchymal stem cells: Prospects for clinical dermatology" J. Am. Acad. Dermatol., vol. 63, pp. 859-865, 2010. [Crossref]
[40] F. Relaix, M. Bencze, M.J. Borok, A. Der Vartanian, F. Gattazzo, D. Mademtzoglou, et al., "Perspectives on skeletal muscle stem cells" Nat. Commun., vol. 12, p. 692, 2021. [Crossref]
[41] W. Lin, L. Xu, S. Lin, L. Shi, B. Wang, Q. Pan, et al., "Characterisation of multipotent stem cells from human peripheral blood using an improved protocol" J. Orthop. Transl., vol. 19, pp. 18-28, 2019. [Crossref]
[42] M.S. Oliveira, J.B. Barreto-Filho, "Placental-derived stem cells: Culture, differentiation and challenges" World J. Stem Cells, vol. 7, pp. 769-775, 2015. [Crossref]
[43] S. Nae, I. Bordeianu, A.T. Stăncioiu, N. Antohi, "Human adipose-derived stem cells: Definition, isolation, tissue-engineering applications" Rom. J. Morphol. Embryol., vol. 54, pp. 919-924, 2013. [PubMed]
[44] J. Li, S.-Q. Xu, Y.-M. Zhao, S. Yu, L.-H. Ge, B.-H. Xu, "Comparison of the biological characteristics of human mesenchymal stem cells derived from exfoliated deciduous teeth, bone marrow, gingival tissue, and umbilical cord" Mol. Med. Rep., vol. 18, pp. 4969-4977, 2018. [Crossref]
[45] M. Bozorgmehr, S. Gurung, S. Darzi, S. Nikoo, S. Kazemnejad, A.-H. Zarnani, et al., "Endometrial and Menstrual Blood Mesenchymal Stem/Stromal Cells: Biological Properties and Clinical Application" Front. Cell Dev. Biol., vol. 8, 2020. [Crossref] [PubMed]
[46] M. Najar, G. Raicevic, T. André, H. Fayyad-Kazan, K. Pieters, D. Bron, et al., "Mesenchymal stromal cells from the foreskin: Tissue isolation, cell characterization and immunobiological properties" Cytotherapy, vol. 18, pp. 320-335, 2016. [Crossref] [PubMed]
[47] "Clinical Trials" Available online: https://www.news-medical.net/news/20240807/New-stem-cell-therapy-for-heart-failure-undergoing-US-clinical-trials.aspx. (accessed on 23 December 2024)
[48] R. Vij, K.A. Stebbings, H. Kim, H. Park, D. Chang, "Safety and efficacy of autologous, adipose-derived mesenchymal stem cells in patients with rheumatoid arthritis: A phase I/IIa, open-label, non-randomized pilot trial" Stem Cell Res. Ther., vol. 13, p. 88, 2022. [Crossref]
[49] Y. Park, Y.J. Lee, J.H. Koh, J. Lee, H.-K. Min, M.Y. Kim, et al., "Clinical Efficacy and Safety of Injection of Stromal Vascular Fraction Derived from Autologous Adipose Tissues in Systemic Sclerosis Patients with Hand Disability: A Proof-Of-Concept Trial" J. Clin. Med., vol. 9, 2020. [Crossref]
[50] D.C. Chambers, D. Enever, N. Ilic, L. Sparks, K. Whitelaw, J. Ayres, et al., "A phase 1b study of placenta-derived mesenchymal stromal cells in patients with idiopathic pulmonary fibrosis" Respirology, vol. 19, pp. 1013-1018, 2014. [Crossref]
[51] S. Al-Ghadban, M. Artiles, B.A. Bunnell, "Adipose Stem Cells in Regenerative Medicine: Looking Forward" Front. Bioeng. Biotechnol., vol. 9, 2022. [Crossref]
[52] Z. Zhang, X. Yang, X. Cao, A. Qin, J. Zhao, "Current applications of adipose-derived mesenchymal stem cells in bone repair and regeneration: A review of cell experiments, animal models, and clinical trials" Front. Bioeng. Biotechnol., vol. 10, 2022. [Crossref]
[53] Q. Jin, K. Yuan, W. Lin, C. Niu, R. Ma, Z. Huang, "Comparative characterization of mesenchymal stem cells from human dental pulp and adipose tissue for bone regeneration potential" Artif. Cells Nanomed. Biotechnol., vol. 47, pp. 1577-1584, 2019. [Crossref] [PubMed]
[54] J. Cuenca, A. Le-Gatt, V. Castillo, J. Belletti, M. Díaz, G.M. Kurte, et al., "The reparative abilities of menstrual stem cells modulate the wound matrix signals and improve cutaneous regeneration" Front. Physiol., vol. 9, 2018. [Crossref]
[55] C.S. Chung, N. Fujita, N. Kawahara, S. Yui, E. Nam, R. Nishimura, "A comparison of neurosphere differentiation potential of canine bone marrow-derived mesenchymal stem cells and adipose-derived mesenchymal stem cells" J. Vet. Med. Sci., vol. 75, pp. 879-886, 2013. [Crossref]
[56] Y. Han, X. Li, Y. Zhang, Y. Han, F. Chang, J. Ding, "Mesenchymal Stem Cells for Regenerative Medicine" Cells, vol. 8, 2019. [Crossref]
[57] Y. Chen, H. Huang, G. Li, J. Yu, F. Fang, W. Qiu, "Dental-derived mesenchymal stem cell sheets: A prospective tissue engineering for regenerative medicine" Stem Cell Res. Ther., vol. 13, pp. 1-15, 2022. [Crossref]
[58] F. Naderi, M. Mehdiabadi, F. Kamarehei, "The therapeutic effects of stem cells from human exfoliated deciduous teeth on clinical diseases: A narrative review study" Am. J. Stem Cells, vol. 11, p. 28, 2022. [PubMed]
[59] L. Yin, Y. Zhu, J. Yang, Y. Ni, Z. Zhou, Y. Chen, et al., "Adipose tissue-derived mesenchymal stem cells differentiated into hepatocyte-like cells in vivo and in vitro" Mol. Med. Rep., vol. 11, pp. 1722-1732, 2015. [Crossref] [PubMed]
[60] R. Zhou, Z. Li, C. He, R. Li, H. Xia, C. Li, et al., "Human umbilical cord mesenchymal stem cells and derived hepatocyte-like cells exhibit similar therapeutic effects on an acute liver failure mouse model" PLoS ONE, vol. 9, 2014. [Crossref]
[61] H.J. Lee, J. Jung, K.J. Cho, C.K. Lee, S.G. Hwang, G.J. Kim, "Comparison of in vitro hepatogenic differentiation potential between various placenta-derived stem cells and other adult stem cells as an alternative source of functional hepatocytes" Differentiation, vol. 84, pp. 223-231, 2012. [Crossref]
[62] Z. Si, X. Wang, C. Sun, Y. Kang, J. Xu, X. Wang, et al., "Adipose-derived stem cells: Sources, potency, and implications for regenerative therapies" Biomed. Pharmacother., vol. 114, 2019. [Crossref]
[63] C. Gavin, S. Meinke, N. Heldring, K.A. Heck, A. Achour, E. Iacobaeus, et al., "The Complement System Is Essential for the Phagocytosis of Mesenchymal Stromal Cells by Monocytes" Front. Immunol., vol. 10, 2019. [Crossref] [PubMed]
We use cookies to improve your experience on our site. By continuing to use our site, you accept our use of cookies. Learn more