
APA Style
Darya Nizheharodava, Elizaveta Nazaranka, Natallia Marozava, Galina Ivanchyk, Volha Karnialiuk, Zhanna Kaliadzich, Marina Zafranskaya. (2025). Characteristics of Tumor Infiltrating Lymphocytes in Patients with Benign and Malignant Sinonasal Neoplasms. Cancer Immunology Connect, 1 (Article ID: 0005). https://doi.org/10.69709/CIConnect.2025.133891MLA Style
Darya Nizheharodava, Elizaveta Nazaranka, Natallia Marozava, Galina Ivanchyk, Volha Karnialiuk, Zhanna Kaliadzich, Marina Zafranskaya. "Characteristics of Tumor Infiltrating Lymphocytes in Patients with Benign and Malignant Sinonasal Neoplasms". Cancer Immunology Connect, vol. 1, 2025, Article ID: 0005, https://doi.org/10.69709/CIConnect.2025.133891.Chicago Style
Darya Nizheharodava, Elizaveta Nazaranka, Natallia Marozava, Galina Ivanchyk, Volha Karnialiuk, Zhanna Kaliadzich, Marina Zafranskaya. 2025. "Characteristics of Tumor Infiltrating Lymphocytes in Patients with Benign and Malignant Sinonasal Neoplasms." Cancer Immunology Connect 1 (2025): 0005. https://doi.org/10.69709/CIConnect.2025.133891.
 ACCESS
Research Article
ACCESS
Research Article
Volume 1, Article ID: 2025.0005

Darya Nizheharodava
nzh@tut.by

Elizaveta Nazaranka
el.m.nazarenko@gmail.com

Natallia Marozava
natalim-22@yandex.ru

Galina Ivanchyk
immunology.by@gmail.com

Volha Karnialiuk
olsol70@mail.ru

Zhanna Kaliadzich
janept@list.ru

Marina Zafranskaya
zafranskaya@gmail.com
1 Research Institute of Experimental and Clinical Medicine, Belarusian State Medical University, 220083 Minsk, Republic of Belarus
2 International Sakharov Environmental Institute, Belarusian State University, 220070 Minsk, Republic of Belarus
3 N. N. Alexandrov National Cancer Center of Belarus, Lesnoy, Minsk District, 223040 Minsk Region, Republic of Belarus
4 ENT Center of the Republic of Belarus, Minsk, 220004, Republic of Belarus
* Author to whom correspondence should be addressed
Received: 21 Dec 2024 Accepted: 27 Feb 2025 Available Online: 06 Mar 2025 Published: 21 Mar 2025
Introduction. Tumor infiltrating lymphocytes are a major component of sinonasal neoplasms, actively populating tumor tissue, yet their role remains poorly understood. The aim is to characterize subsets and functional profiles of tumor infiltrating lymphocytes in patients with benign and malignant sinonasal tumors. Materials and methods. Tumor infiltrating lymphocytes were isolated from the biopsy material of 58 patients: 18 with sinonasal malignancies, 23 with inverted papilloma and 17 with polypous rhinosinusitis (control group). Flow cytometry was used for tumor infiltrating lymphocytes immunophenotype estimation. The production of γ-interferon in tissue homogenates was measured by enzyme-linked immunosorbent assay kit. Further, statistical analysis was done using GraphPad Prism 8. Results and discussion. The microenvironment of sinonasal malignancies is characterized by increased CD3+T cells (
Tumors of the nasal cavity and paranasal sinuses are rare (account for 1–3% of malignant tumors of all localizations), and are heterogeneous neoplasms that are characterized by multifactorial etiology. Their development is influenced by genetic predisposition (such as associations with HLA-B17 and HLA-Bw26), environmental factors, and chronic viral infections, including human papillomavirus (HPV) and Epstein-Barr virus (EBV) [1,2,3]. Although tumors of the nasal cavity and paranasal sinuses are presented with different histological features and clinical behavior, squamous cell carcinoma accounts for 90% of head and neck cancer [4]. Inverted papilloma (IP) represents the predominant type of benign tumors in the sinonasal tract [5]. Due to the poverty of prospective and large clinical trials, the main problem is the lack of biomarkers for early detection of the malignant process. Thereby, late diagnosis results in poor effectiveness of treatment and low survival of patients. From a diagnostic point of view, it is mandatory not only to perform an immunohistochemical tumor assessment but also to understand the immunological peculiarities of malignant and benign sinonasal neoplasms. Thereby, the local microenvironment and tumor-infiltrating lymphocytes (TILs) are of high scientific and practical interest. TILs are immune cells that migrate to the area of the primary neoplasm and actively infiltrate it as well. They can also be identified in metastases and regional lymph nodes, making them the objects of increased researcher’s attention worldwide. However, the understanding of TILs role in the pathogenesis of cancer is still poorly investigated [6,7,8]. Initial studies suggested TILs to be cytotoxic to cancer cells, having almost no effect on healthy tissues. Later, the dual role of TILs in the tumor microenvironment was confirmed: on the one hand, TILs can suppress tumor growth by destroying transformed cells or inhibiting their growth, and, on the other hand, TILs promote tumor progression via selecting immune resistant clones or creating conditions in the tumor microenvironment that promote its growth [9,10,11]. Such a wide functional spectrum is primarily due to the heterogeneity of TILs, which mainly include subpopulations of T lymphocytes, and to a lesser extent, B lymphocytes, NK cells and innate lymphoid cells. The same populations of immune cells can exhibit both anti- and pro-tumorigenic effects. Their composition and functional state can vary significantly depending on the tumor type, disease stage, therapy, and the method of cell isolation and cultivation [6,12]. TILs prognostic value is still under debate due to contradictory data. Many authors have demonstrated TILs to correlate with clinical outcome in different malignant neoplasms, and have shown that the degree of tumor infiltration by lymphocytes determines the disease prognosis [13,14,15]. According to some data, TILs are recognized as a more significant predictor of patient’s survival than the TNM classification of malignant tumors [10]. Nonetheless, most of these studies were conducted using immunohistochemical analysis of fixed blocks that allows comparing laboratory and clinical results. However, this methodological approach provides information only about the presence of infiltration, its exact localization and severity, but does not characterize the subpopulation composition and TILs functional profile. Disadvantages of the current methodology for TILs determination as a prognostic biomarker include the lack of quantitative immuno(cyto)histochemistry approaches, insufficient reproducibility of results, and labor-intensive procedures for TILs measuring that can be improved by using the flow cytometry. Thus, the determination of the immunological characteristics of benign and malignant sinonasal neoplasms is a highly relevant area that will allow clarifying the specific contribution of immune parameters to systematization and timely diagnostics of sinonasal malignant and benign tumors. In this article, the characteristics of TILs subsets and function profile in patients with sinonasal neoplasms is presented for the first time, aimed at assessing the TILs differences in malignant and benign tumor-associated pathological process in the nasal cavity and paranasal sinuses.
Patients. The biopsy material was obtained from 58 patients (37 men and 21 women, average age of 56.5 (43.5; 64.3) y.o.) hospitalized at N.N.Alexandrov National Cancer Centre of Belarus or the Republican Scientific and Practical Center of Otorhinolaryngology from January 2022 to December 2024. All subjects were divided into three groups: group 1–18 patients with sinonasal malignant neoplasms; group 2–23 patients with IP; group 3–17 patients with polypous rhinosinusitis (PRS) as a control group. The group of malignant tumors of the nasal cavity and paranasal sinuses included patients with the following diagnoses: squamous cell carcinoma (n = 10), melanoma (n = 5), sarcoma (n = 2), neuroendocrine cancer (n = 1). Clinical and demographic characteristics of patients are presented in Table 1. Clinical and demographic characteristics of patients. Notes: group 1—patients with sinonasal malignant tumors; group 2—patients with inverted papilloma; group 3—patients with polypous rhinosinusitis; Me (Q25; Q75)—median (lower quartile; upper quartile); n—number; negative localization—location of pathological focus in sphenoid sinus, orbital or cranial cavity; T—refers to the tumor size and extent in TNM system for classifying a malignancy; n.d.—not determined. TILs isolation protocol. Biopsy materials were routinely processed within 1–2 h of surgical excision using standard biosafety procedures for human tissues. Sinonasal tumors were weighed, minced into small fragments of 2–4 mm, transferred into the gentleMACS C tube («Miltenyi Biotec», Bergisch Gladbach, Germany) containing 4.7 mL RPMI-1640 («Bio-Whittaker», Walkersville, MD, USA) along with an enzyme mix of 200 μL enzyme H, 100 μL enzyme R, and 25 μL enzyme A (Human Tumor Dissociation Kit, «Miltenyi Biotec», Bergisch Gladbach, Germany) per 0.2–1.0 g of tumor sample. The samples then underwent enzymatic tissue digestion using the gentleMACS Dissociator («Miltenyi Biotec», Bergisch Gladbach, Germany). The dissociation was carried out by running the gentleMACS program «h_tumor_01» followed by samples incubation for 30 min at 37 °C under continuous rotation. Cell suspension was applied onto a 70 μm cell strainer («Millipore», Burlington, MA, USA) and washed with 20 mL of RPMI-1640 at 300 g for 7 min for further immunophenotyping or cell culturing. Flow cytometry method. The immunophenotype of TILs basic subsets was determined using the next CYTO-STAT tetraCHROME monoclonal antibody panels: CD45-FITC/CD4-RD1/CD8-ECD/CD3-PC5 and CD45-FITC/CD56-RD1/CD19-ECD/CD3-PC5 («Beckman Coulter», Indiana, IN, USA). γδT lymphocytes subsets were identified using a DuraCloneIMTCRs monoclonal antibody panel: γδTCR-FITC/αβTCR-PE/Vδ1TCR-PC7/CD4-APC/CD8-AF700/CD3-AF750/Vδ2TCR-РB/CD45-KrO («Beckman Coulter», Indiana, IN, USA). CD19+CD5+B1 lymphocytes were estimated using CD45-FITC/CD5-PE/CD19-ECD panel («Beckman Coulter», Indiana, IN, USA). Regulatory T cells (Treg) were detected as CD3+CD4+CD127lowCD25hi cells using CD127-FITC/CD25-PE/CD4-PC7/CD3-APC/CD45-PB panel («Beckman Coulter», Indiana, IN, USA). Programmed cell death 1 (PD-1)-positive or CD16-positive cells were measured with CD4-FITC/CD279-PE/CD8-ECD/γδTCR-PC7/CD3-APC/CD45-PB or CD3-FITC/CD56-PE/CD16-ECD/γδTCR-PC7/CD45-PB panels, respectively («Beckman Coulter», Indiana, IN, USA; «BioLegend», San Diego, CA, USA). Monoclonal antibody reagents were added according to the manufacturer’s instructions to 100 μL of TILs suspension, and reaction mixtures were incubated at 20–25 °C for 15 min in the dark. Results were measured using a 10-channel Cytoflex flow cytometer («Beckman Coulter», Brea, CA, USA) and analyzed on 10,000 CD3+T lymphocytes or 1,000 γδT lymphocytes using CytExpert software (version 2.3.0.84, «Beckman Coulter», Brea, CA, USA). The intracellular γ-interferon (γIFN) and cytotoxic T lymphocyte-associated protein 4 (CTLA-4) production was evaluated in 24 h TILs cultures in RPMI-1640 medium («Bio-Whittaker», USA) completed with 10% fetal calf serum («Gibco», Waltham, MA, USA), 2 mM L-glutamine («Bio-Whittaker», Walkersville, MD, USA), 1% antibiotic-antimycotic («Gibco», Waltham, MA, USA) in CO2-incubator («Thermo Fischer Scientific», Waltham, MA, USA) with 5% CO2 and 37 °C. For quantitative intracellular γIFN and CTLA-4 determination 4 ng/mL phorbol 12-myristate 13-acetate («Sigma», Darmstadt, Germany), 1 μg/mL of ionomycin calcium salt and 10 μg/mL brefeldin A («Cayman Chemicals», Ann Arbor, MI, USA) were added in the last 4 h of cell culture activation. Then, TILs were stained with monoclonal antibodies panel CD3-FITC/CD8-PC5/γδTCR-PC7/CD45-PB («Beckman Coulter», Indiana, IN, USA) at 20–25 °C for 15 min in the dark, fixed with 4% paraformaldehyde («Sigma», Darmstadt, Germany), permeabilized with 2% Triton X («Sigma», Darmstadt, Germany). Intracellular staining was performed using monoclonal antibodies γIFN-PE or CTLA-4-PE («Beckman Coulter», Indiana, IN, USA). The results were analyzed on 10,000 CD3+T lymphocytes or 1,000 γδT lymphocytes using flow cytometer. ELISA. γIFN concentration was measured in supernatants of tissue homogenates using «gamma-Interferon-ELISA» kit (А-8752, «Vector-Best», Novosibirsk, Russian Federation, diagnostic sensitivity—2.0 pg/mL) according to the manufacturer’s manual. For tissue homogenates preparation tissue pieces were weighed and then homogenized in phosphate buffered saline (PBS) as tissue weight (g): PBS (mL) volume = 1:9 using homogenization program in gentleMACS Dissociator («Miltenyi Biotec», Bergisch Gladbach, Germany). To further break down the cells, the suspensions were subjected to freeze-thaw cycles. Then, homogenates were centrifuged for 5–10 min at 5000× Statistical method. Statistical data processing was performed using GraphPad Prism 8 («GraphPad Software Inc.», Boston, MA, USA). Data were tested for normality with a Shapiro-Wilk test. The median (Ме), lower (Q25) and upper (Q75) quartiles were used as descriptive statistics of the studied groups. Significant differences between investigated groups were determined by nonparametric Kruskal-Wallis test with Dunn post hoc test. Significance levels were set at
Parameters
Group 1
(n = 18)Group 2
(n = 23)Group 3
(n = 17)
Age, years, Me (Q25; Q75)
59
(49; 66)53
(38; 67)56
(45; 63)
Gender, n (%)
male
11 (61.1)
16 (69.6)
10 (58.8)
female
7 (38.9)
7 (30.4)
7 (41.2)
Smoking, n (%)
yes
4 (22.2)
7 (30.4)
4 (23.5)
no
14 (77.8)
16 (69.6)
13 (76.5)
Bleeding, n (%)
yes
5 (27.8)
3 (13.0)
1 (5.9)
no
13 (72.2)
20 (87.0)
16 (94.1)
Anosmia, n (%)
yes
4 (22.2)
8 (34.8)
10 (58.8)
no
14 (77.8)
15 (65.2)
7 (41.2)
Ophthalmological symptoms, n (%)
yes
6 (33.3)
1 (4.3)
2 (11.8)
no
12 (66.7)
22 (95.7)
15 (88.2)
Negative localization, n (%)
yes
7 (38.9)
2 (8.7)
6 (35.3)
no
11 (61.1)
21 (91.3)
11 (64.7)
Fibrinogen, g/L, Me (Q25; Q75)
3.62
(3.05; 4.25)3.15
(2.97; 4.60)3.17
(2.75; 3.61)
T classification,
n (%)T0
2 (11.1)
n.d.
n.d.
T1
3 (16.7)
T2
1 (5.5)
T3
2 (11.1)
T4, n (%)
10 (55.6)
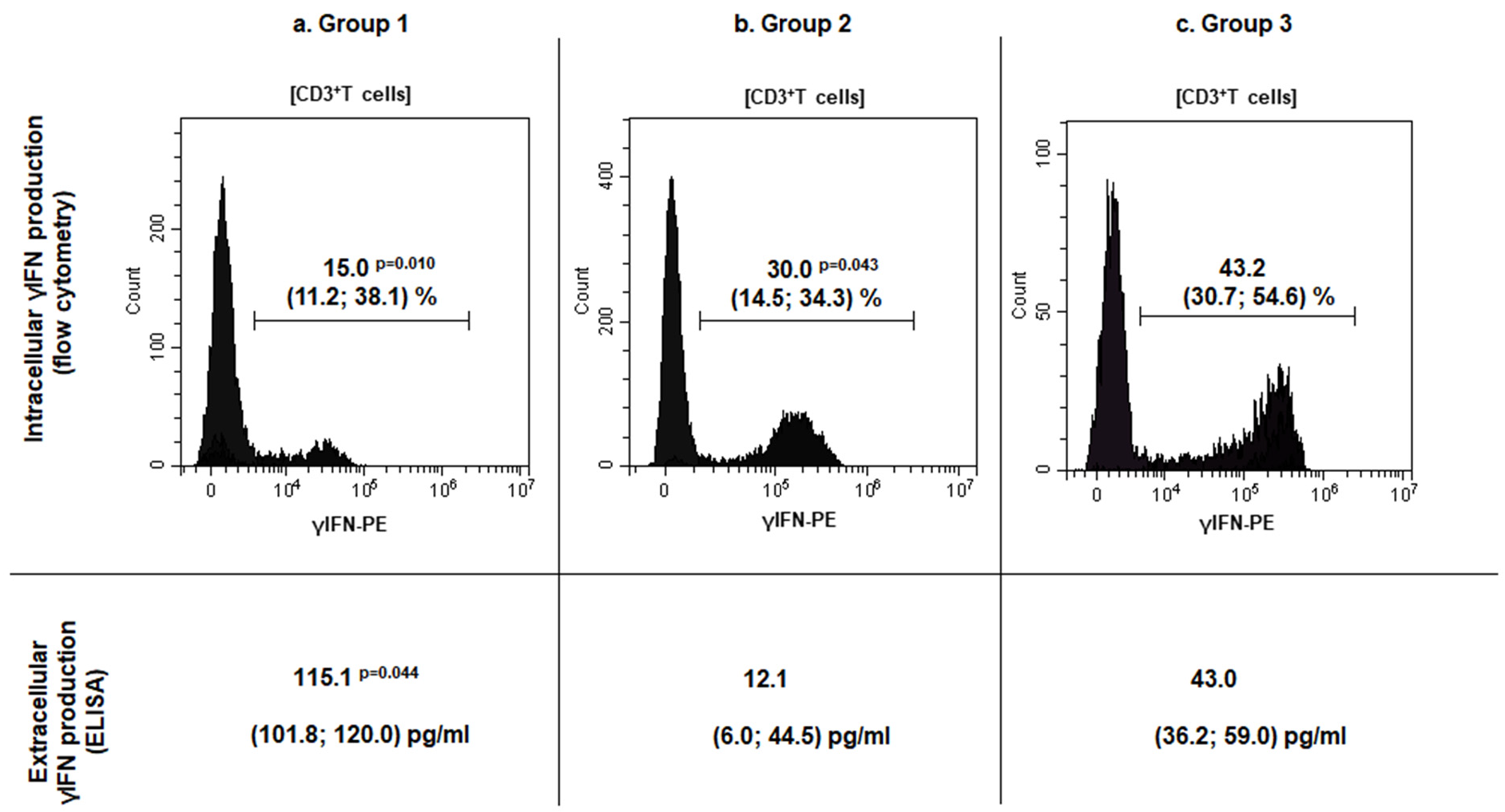
TILs number and subsets characteristics in patients with sinonasal tumors. A comparative analysis of TILs concentration and phenotype was performed in investigated groups. A significant increase in TILs number per 1 g of tissue was detected in both patient groups with malignant sinonasal neoplasms, and IP as compared to patients with PRS: 23.6 (11.7; 28.5) × 106 (group 1, The obtained results were consistent with the data of other authors, stating an increased lymphoid infiltration of sinonasal neoplasm tissue, along with the number of migrating cells into the lesion. This reflected the active involvement of the cellular immune compartment in the anti-tumor response [6,10,16]. Many studies have described not only TILs infiltration in the head and neck squamous cell carcinoma but also their correlation with outcome, since Wolf first had documented it in 1986 [11,14,15,17]. However, conflicting data have been reported about TILs association with improved prognosis that can be explained by differences in subsets, tumor compartment localization (intra-tumoral versus stromal) and HPV-positive/negative patients’ status [7]. Thus, further progression of sinonasal neoplasms is largely determined by the TILs subpopulation composition, the functional profile of which can be characterized by both pro- and anti-tumorigenic effects. To characterize TILs subsets in patients with sinonasal tumors, the relative numbers of the major and minor populations of T, B and NK cells among TILs were investigated. The results are presented in Figure 1. Increased CD3+TILs (Figure 1a, p = 0.003) in combination with decreased CD19+TILs (Figure 1e, The investigation of minor lymphoid cells subsets revealed increased Treg numbers in patients with malignant and benign tumors, as compared to the control group (Figure 1i, respectively, p = 0.044 and Meanwhile, there were no significant differences in the percent of B1 cells (Figure 1f) and γδT cells (Figure 1d) among TILs of the investigated groups. However, the assessment of γδT cells subsets based on Vδ repertoire of T cell receptors (tissue-resident Vδ1+ or Vδ1–Vδ2–T cells and circulating Vδ2+T cells) showed changes: in group 1, Vδ2+T cells subset prevailed among others (44.3 (27.3; 53.4)%, p = 0.001), while the dominated subsets in group 2 and 3 were Vδ1+T cells (54.3 (27.1; 70.4) % and 40.1 (33.8; 49.0) %, respectively, Based on the prevalence of immune cells inside and outside the tumor, Kather et al. assessed a domination of different immune cells in various cancer types and classified the tumors into three groups i.e., «cold» (presence of fewer immune cells both outside and inside the tumor), «immune excluded» (presence of fewer immune cells inside and more immune cells outside of the tumor), and «hot» (presence of more immune cells inside regardless immune cell density outside) [18]. According to the data, the identification of CD3+T lymphocytes absolute majority among TILs, in combination with up-regulated tissue-resident Treg in patients with malignancies, corresponded to Kather et al., who had established a high incidence of CD3-hot, CD8-hot, FOXP3-hot and PD-1-hot tumors in head and neck squamous carcinoma. In contrast to a positive outcome in patients with active TILs infiltration, meta-analysis data indicate that FOXP3+Treg in tumor tissue predicted worse survival rates in the majority of solid tumors, including head and neck cancers [19]. Moreover, according to Lou et al., high levels of CD8+TILs infiltration were related to poor differentiation and lymph node metastases in patients with sinonasal squamous cell carcinoma. The paradox of CD8+TILs and tumor progression coexistence may be explained by a complex crosstalk between tumor and infiltrating immune cells [20]. In contrast, Yin et al. demonstrated that CD8+T cells and TNK cells were highly expressed in patients with no sinonasal mucosal melanoma progression, while Th2 T cells, macrophages and M2 macrophages were highly expressed in patients with disease progression [21]. Liu et al. reported that HPV-positive patients with head and neck squamous cell carcinoma exhibit higher expression of CD8+T cells, Treg, dendritic cells, and CD56dimNK cells [22]. The human immune system uses a wide range of cellular factors and molecules to implement mechanisms of anti-tumor immunity, resulting in effective destruction of tumors. The main mechanisms are contact killing mediated CD8+T lymphocytes or NK/TNK cells, as well as antibody-dependent cellular cytotoxicity. Upon activation and recognition of tumor cells, cytotoxic lymphocytes can induce tumor cells death via various pathways, including perforin/granzyme B, FasL-Fas, TRAIL, or inflammatory cytokines. Another effector mechanism is mediated by antibodies, binding to which results in tumor cells destruction via activating the complement cascade, or the transmission signals through Fc receptors on immune cells. It results in antibody-dependent cellular phagocytosis or cellular cytotoxicity involving neutrophils and NK cells [1,6,23]. Due to the fact that the tumor microenvironment forms an unfavorable surrounding, and cells are immature or tolerant, it is crucial to investigate not only the composition of cell subsets but also the functional changes in anti-tumor effector mechanisms. In this regard, TILs functional profile, including the estimation of the cytotoxic innate and adaptive TILs potential, as well as mechanisms of TILs silencing were further investigated in patient groups. The characteristics of effector cytotoxicity mechanisms of immune killer TILs in patients with sinonasal tumors. To assess the effector mechanisms of specific immunity in patients with sinonasal tumors, the intra- and extracellular γIFN production (Figure 2) as well as CD16 (Fc receptor to IgG fragment) expression (Table 2) as key regulators of anti-tumor immunity were characterized. CD16- and γIFN-positive TILs subsets and tissue γIFN production in patients with sinonasal tumors, Me (Q25; Q75). Notes: TILs—tumor-infiltrating lymphocytes; group 1—patients with sinonasal malignant tumors; group 2—patients with inverted papilloma; group 3—patients with polypous rhinosinusitis; Me (Q25; Q75)—median (lower quartile; upper quartile). Significant p-values are shown in bold. A down-regulation of intracellular γIFN production in CD3+TILs of patients with malignant neoplasms was determined as compared to group 3 (p = 0.010). A similar decrease in the percentage of CD3+γIFN+TILs in the local microenvironment was detected in patients with benign tumors as compared to group 3 ( Thus, the microenvironment of sinonasal malignant tumors is characterized by a high TILs level as well as extracellular γIFN synthesis in tissue, along with a markedly decreased population of γIFN-producing T cells. This may reflect the activation of the tumor-specific immune response and suggest that T cells fully exert their effector γIFN-mediated potential at the site of the pathological process. This can lead to failure due to immunological depletion of cytotoxic T lymphocytes and decreased reserve capacity of cellular immunity. Kondoh et al. have also obtained similar results, reporting a high γIFN-producing capability among patients with oral squamous cell carcinoma at stage I, but it was decreased among patients whose tumor progressions were at stages II and III [24]. Norouzian et al. have demonstrated that larger tumor size, higher stage of the disease and/or lymph nodes involvement were associated with lower frequencies of CD8+γIFN+T cells [25]. In patients with IP, a decrease in intracellular γIFN production, in association with normal CD3+TILs and extracellular γIFN level at the site of the tumor may indicate insufficient stimulation of effector reactions, mediated by type II interferon from antigen-presenting cells. This mechanism requires further detailed study at the level of induction and regulation mechanisms of interferon synthesis. In addition to its autocrine effect on primary γIFN producing cells, this cytokine can also influence stromal cells in the inflammatory or tumor environment, such as macrophages, myeloid-derived suppressor cells, dendritic cells, and B cells. γIFN acts as a cytotoxic cytokine together with granzyme B and perforin initiating apoptosis in tumor cells, but, on the other hand, it can also promote the synthesis of PD-L1 and indoleamine 2,3-dioxygenase, thereby stimulating other immunosuppressive mechanisms [24,25,26]. One of the actively studied areas in cancer immunological research is assessment of expression of CD16 receptors on subpopulations of lymphoid cells. Low-affinity receptors type III of immunoglobulin G Fc-fragments (CD16, FCγR3) are predominantly expressed as CD16A protein (FCγR3A) on NK cells, and may be found on the membrane of monocytes, tissue-specific macrophages, γδT lymphocytes and dendritic cells. The presence of CD16A on TNK and γδT lymphocytes allows these cells to recognize tumor cells without MHC I, unlike T killers, and to carry out antibody-dependent cellular cytotoxicity due to the presence of the Fc receptor on their membrane. Evaluation of membrane CD16 expression is used to determine the population composition and their functional status in various diseases, including oncology [27,28]. In this regard, the expression of the CD16 receptor on subpopulations of lymphoid cells in the tumor tissue was assessed in patients with sinonasal tumors (Table 2). Decreased CD56+CD16+NK cells among TILs in tumor tissue of patients with sinonasal malignancies were shown, as compared to patients with PRS (p = 0.027). While there were no significant differences in CD16 expression on CD3+TILs and γδTCR+TILs in group 1 (Table 2). The results indicate the failure of antibody-dependent cytotoxicity at the level of innate immune cells, contributing to the protumorigenic pathogenesis of neoplasms. Decreased antibody-dependent cellular cytotoxicity is likely to occur under the influence of inhibitory receptors in the tumor microenvironment. To our knowledge, no studies have yet been published on the specific function of CD16+T cells in sinonasal malignancies. However, Zöphel et al. have found that the percentage of CD16+T cells (>1.6% CD16+ T cells) is a protective predictor for the progression-free survival of patients with non-Hodgkin B cell lymphoma over a 24-month period [29]. Meanwhile, Lalos et al. reported that a high density of CD16-expressing TILs in recurrent ovarian cancer is associated with improved recurrence-free survival and overall survival [30]. The expression of CTLA-4 and PD-1 on TILs in patients with sinonasal tumors. Immune checkpoint inhibitory molecules are a system of regulatory mechanisms acting through the activation of immunosuppressive signaling, resulting in the formation of peripheral tolerance and promoting the development of neoplasms [22,31]. The expression of most studied checkpoint inhibitory molecules—cytotoxic T lymphocyte associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1)—was investigated on TILs membrane in patients with sinonasal tumors. The results are presented in Table 3. PD-1- and CTLA-4-positive TILs subsets in patients with sinonasal tumors, Me (Q25; Q75). Notes: TILs—tumor-infiltrating lymphocytes; PD-1—programmed cell death 1; CTLA-4—cytotoxic T-lymphocyte-associated protein 4; group 1—patients with sinonasal malignant tumors; group 2—patients with inverted papilloma; group 3—patients with polypous rhinosinusitis; Me (Q25; Q75)—median (lower quartile; upper quartile). Significant p-values are shown in bold. Increased CTLA-4 expression on CD3+TILs was established in both groups of patients with sinonasal tumors, malignant (p = 0.036) as well as benign ( There were no significant differences in the expression of the checkpoint inhibitory molecule PD-1 on CD3+TILs and their subsets across all patient groups (Table 3), which contradicts the findings of some authors [11,18,28]. PD-1 is a membrane protein of the immunoglobulin superfamily that negatively regulates T cell activation by inducing dephosphorylation and inactivation of the T cell kinase ZAP70, thereby reducing the production of inflammatory cytokines and cell survival proteins [35]. Along with CTLA-4, PD-1 represents a promising target for the development of new therapeutic strategies for anticancer therapy, but its role in sinonasal malignancies is still controversial. The correlation of clinical and immunological parameters in patients with sinonasal tumors. To identify potential predictors for possible malignancy of the tumor, a correlation analysis of TILs number and their functional profile with patients’ clinical parameters including smoking, bleeding, anosmia, ophthalmological symptoms, tumor size and extent (T) was performed. The results are presented in Figure 3. In patients of group 1, the number of CD3+TILs correlated with negative localization (R = 0.53; p = 0.021) and serum fibrinogen level (R = 0.56, 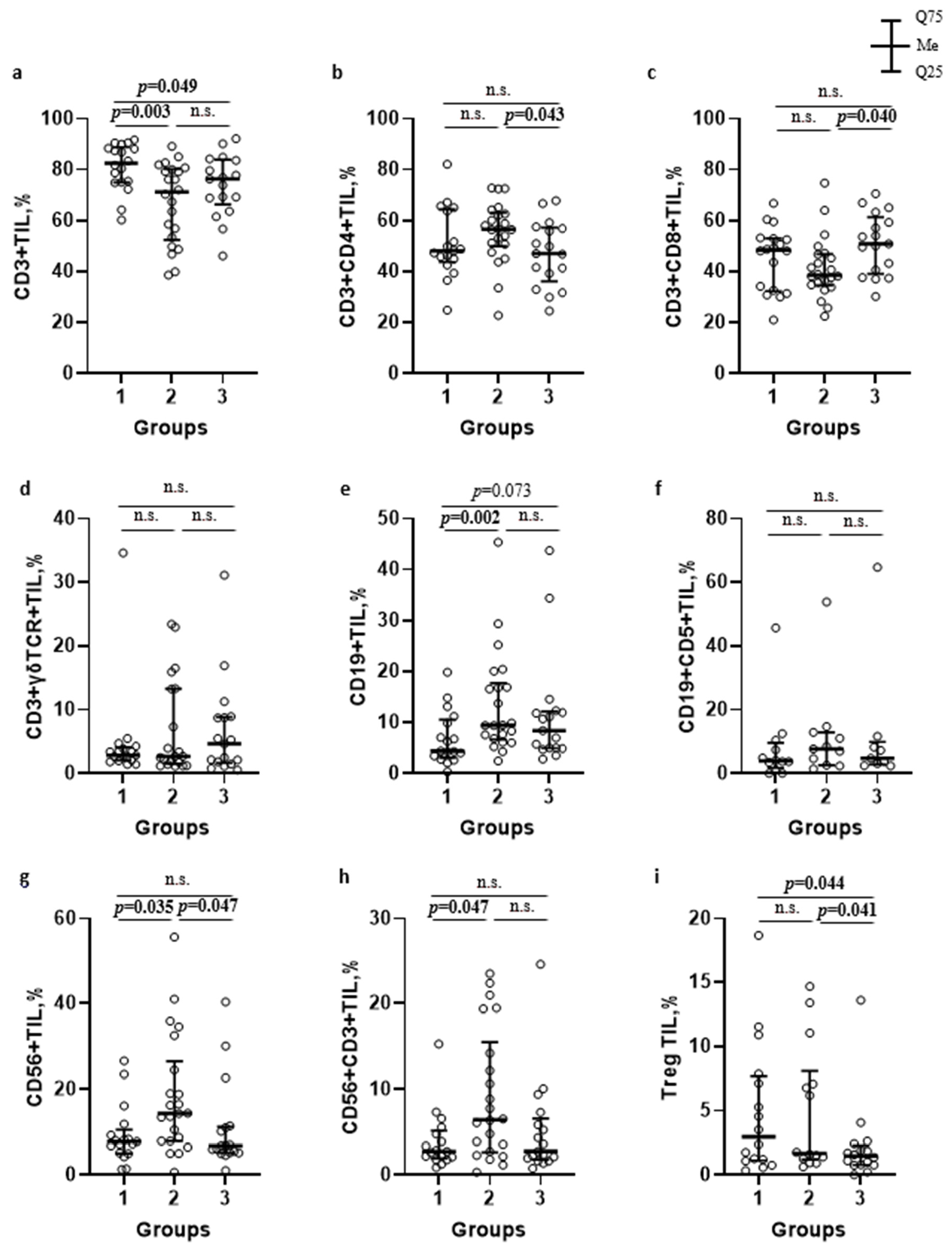
TILs Subsets
Patients’ Groups
р-Value
Group 1
Group 2
Group 3
Kruskal-Wallis Test
Dunn Post-Hoc Test
CD16 + cells
CD3+CD16+
TILs, %1.4
(0.5; 2.7)1.6
(0.7; 7.8)3.0
(1.1; 5.3)p = 0.098
p1-2 = 0.832
p1-3 = 0.094
p2-3 > 0.999
γδТCR+CD16+
TILs, %15.4
(4.5; 23.4)25.3
(9.6; 34.0)12.1
(9.5; 16.4)p = 0.383
p1-2 = 0.803
p1-3 > 0.999
p2-3 = 0.587
CD56+CD16+
TILs, %20.7
(17.5; 40.4)27.0
(21.8; 45.1)39.2
(32.0; 58.2)
p =
0.031
p1-2 > 0.999
p1-3 = 0.027
p2-3 = 0.386
TILs Subsets
Patients Groups
р-Value
Group 1
Group 2
Group 3
Kruskal-Wallis Test
Dunn Post-Hoc Test
CTLA-4+ cells
CD3+CTLA-4+
TILs, %43.0
(29.2; 56.8)36.7
(34.2; 45.1)23.6
(17.2; 43.1)
p =
0.019
p1-2 > 0.999
p1-3 = 0.036
p2-3 = 0.047
CD4+CTLA-4+
TILs, %55.0
(39.8; 68.1)55.4
(49.4; 62.2)34.2
(29.3; 51.2)p = 0.013
p1-2 > 0.999
p1-3 = 0.049
p2-3 = 0.018
CD8+CTLA-4+
TILs, %26.9
(15.1; 33.4)13.6
(7.8; 35.1)13.1
(7.9; 17.5)
p =
0.039
p1-2 = 0.450
p1-3 = 0.033
p2-3 = 0.616
γδТCR+CTLA-4+
TILs, %44.3
(25.6; 59.2)53.7
(36.4; 70.3)31.2
(20.8; 44.9)
p =
0.023
p1-2 = 0.332
p1-3 = 0.865
p2-3 = 0.021
PD-1+ cells
CD3+PD-1+
TILs, %80.4
(71.4; 85.8)70.3
(57.2; 87.8)77.2
(72.8; 82.7)p = 0.646
p1-2 > 0.999
p1-3 > 0.999
p2-3 > 0.999
CD4+PD-1+
TILs, %80.6
(60.6; 84.8)68.2
(58.5; 81.8)76.4
(66.1; 81.6)p = 0.701
p1-2 > 0.999
p1-3 > 0.999
p2-3 > 0.999
CD8+PD-1+
TILs, %75.9
(69.2; 85.8)77.8
(60.6; 85.1)80.9
(72.6; 86.3)p = 0.617
p1-2 > 0.999
p1-3 > 0.999
p2-3 = 0.980
γδТCR+PD-1+
TILs, %71.4
(57.6; 87.6)66.4
(48.9; 87.2)80.2
(60.3; 83.9)p = 0.862
p1-2 > 0.999
p1-3 > 0.999
p2-3 > 0.999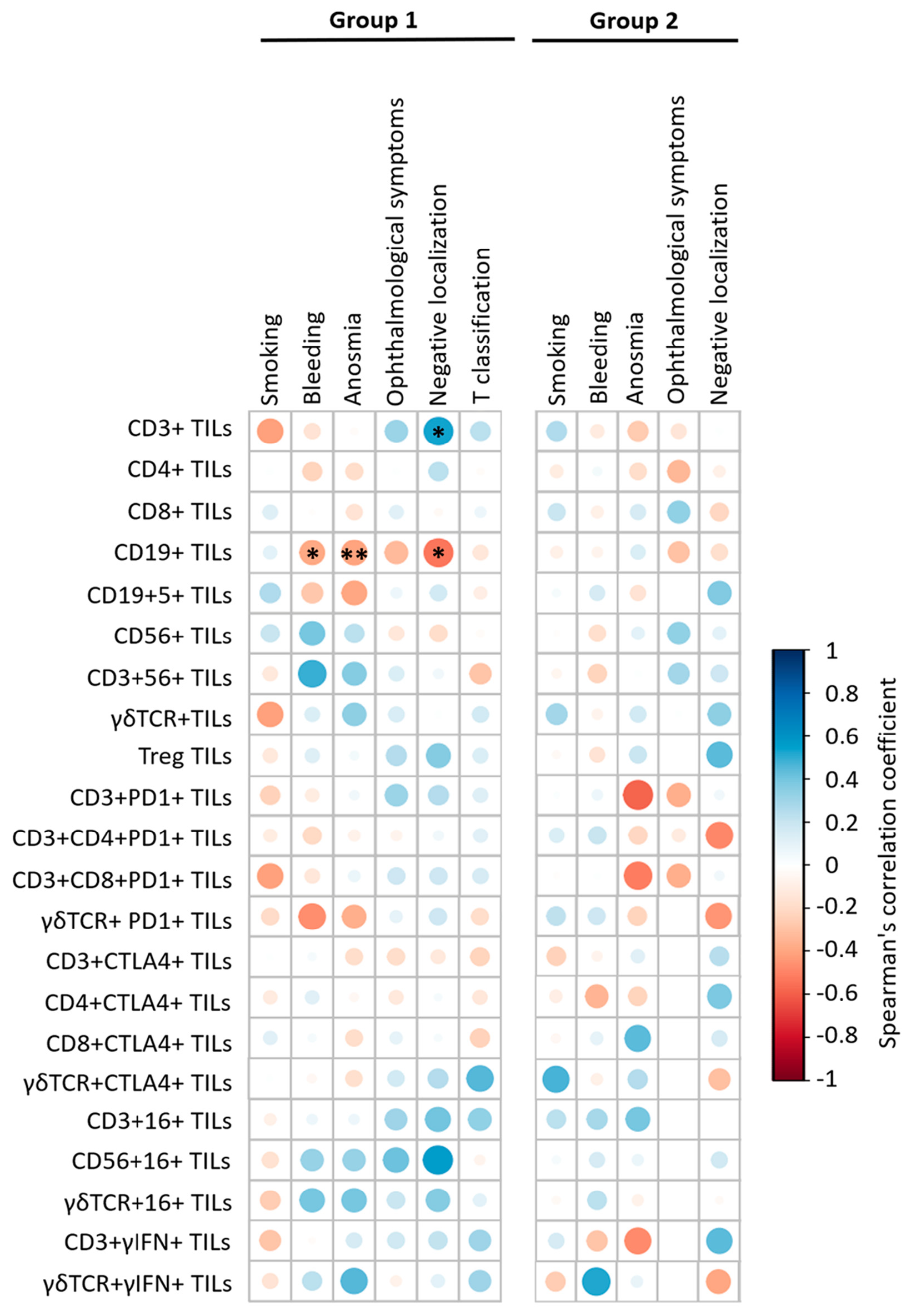
The present study is associated with some limitations. Firstly, this was a single-center retrospective study with a limited number of patients and a heterogeneous group of malignancies. On the other hand, this study was preliminary and paves the way towards further investigation, including prospective studies with a larger number of more homogeneous groups, and broader clinical sample validation that is necessary to confirm prognostic significance of immunological biomarkers. Secondly, the study did not assess the clinical importance of the association between TILs subsets and their localization in tumor tissue using hematoxylin-eosin stained sections. However, the International TILs Working Group does not currently recommend that immunohistochemistry be used to detect specific subpopulations. In the near future, this association will be clarified. Thirdly, many mechanisms for immunosuppressive tumor microenvironment are already known, but the study investigated only γIFN- and CD16-mediated TILs effector anti-tumor mechanisms, as well as role of checkpoint inhibitory molecules CTLA-4 and PD-1 in immunological tolerance formation. Therefore, further comprehensive research is needed to better understand cytotoxic mechanisms in sinonasal tumors, including TILs functions exhaustion, not only at the level of immune cells, but also tumor cells.
TILs play a central role in the surveillance of malignant and benign sinonasal neoplasms. However, currently the generally accepted approach to the immune status assessment focuses on the characterization of peripheral blood lymphoid cells, and the phenotypic and functional TILs characteristics are still not fully investigated. In this study, the involvement of various mechanisms of cellular cytotoxicity and tolerance formation to tumor antigens has been demonstrated in patients with benign and malignant sinonasal neoplasms. The microenvironment tissue in patients with malignant sinonasal tumors is accompanied by decreased TILs cytotoxicity mechanisms, due to FcRγ (CD16) down regulation on NK cells and T lymphocytes, along with increased Treg and CTLA-4-positive CD3+TILs. The cytokine profile of sinonasal malignancies is characterized by a significantly high level of local extracellular γIFN production, in combination with a pronounced decrease in the number of γIFN-producing T cells. This indicates the implementation of the effector γIFN-mediated potential by T cells in the focus of the pathological process, potentially leading to immune response failure as a result of cytotoxic T lymphocytes immunological depletion and a decreased reserve capacity of cellular immunity. Decreased CD16 expression on NK cells and increased CTLA-4 expression only on T helper TILs subset have been established in IP patients. The detected significant decrease in both intracellular and extracellular γIFN production in the tumor tissue may indicate insufficient stimulation of γIFN-mediated effector reactions in patients with a benign neoplasm. The application of tissue resident Treg, CTLA-4- and CD16-positive CD3+TILs, as well as intracellular γIFN production may be considered as potential prognostic biomarkers for malignancy in sinonasal tumors and requires further investigation.
CD
Cluster of Differentiation
CTLA-4
Cytotoxic T Lymphocyte Associated Protein 4
FasL
Fas Ligand
Fas
Fas Receptor
FOXP3
Forkhead Box P3
Fc
Crystallisable Fragment of Immunoglobulin
HLA
Human Leukocyte Antigen
HPV
Human Papillomavirus
γIFN
γ-Interferon
IgG
Immunoglobulin G
IP
Inverted Papilloma
MHC
Major Histocompatibility Complex
NK
Natural Killer
PD-1
Programmed Cell Death 1
PD-L1
Programmed Cell Death Ligand 1
PRS
Polypous Rhinosinusitis
TCR
T-Cell Receptor
TILs
Tumor-Infiltrating Lymphocytes
TNK
Natural Killer T Cells
TRAIL
Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand
Treg
Regulatory T Cells
ZAP-70
Zeta-Chain-Associated Protein Kinase 70
M.Z., D.N. and Z.K. designed the study; D.N. and M.Z. drafted the manuscript; N.M., V.K. and Z.K. performed surgical procedures; D.N., E.N., G.I. and M.Z. carried out tissue samples proceeding and immunological experiments; D.N., E.N. and M.Z. analyzed and discussed the data and generated the figures and tables. All authors have read and agreed to the published version of the manuscript.
Data supporting the results of this study are available upon request from the corresponding author.
Not applicable.
The authors declare that they have no conflicts of interest.
The funding for this research was provided by the Ministry of Health of Republic of Belarus, grant number 20221348.
The study was approved by the Ethics Committee of N. N. Alexandrov National Cancer Center of Belarus (protocol No. 17 dated 07.06.2022) and all patients provided written informed consent to participate in the study.
The study was performed following the Declaration of Helsinki (1975, revised in 2013).
[1] J. Schoenfeld, "Immunity in head and neck cancer" Cancer Immunol. Res., vol. 3, pp. 12-17, 2015. [Crossref] [PubMed]
[2] J.G. Eide, K.C. Welch, J.N. Adappa NDPalmer, C.C. Tong, "Sinonasal Inverted Papilloma and Squamous Cell Carcinoma: Contemporary Management and Patient Outcomes" Cancers, vol. 14, 2002. [Crossref] [PubMed]
[3] A. Skálová, A. Agaimy, M. Bradova, V.V. Poorten, E. Hanna, O. Guntinas-Lichius, et al., "Molecularly defined sinonasal malignancies: An overview with focus on the current WHO classification and recently described provisional entities" Virchows Arch., vol. 484, pp. 885-900, 2024. [Crossref]
[4] M. Gormley, G. Creaney, A. Schache, K. Ingarfield, D.I. Conway, "Reviewing the epidemiology of head and neck cancer: Definitions, trends and risk factors" Br. Dent. J., vol. 233, pp. 780-786, 2022. [Crossref]
[5] H. Lou, J. Fang, P. Li, W. Zhou, Y. Wang, E. Fan, et al., "Frequency, Suppressive Capacity, Recruitment and Induction Mechanisms of Regulatory T Cells in Sinonasal Squamous Cell Carcinoma and Nasal Inverted Papilloma" PLoS ONE, vol. 10, 2015. [Crossref]
[6] S.T. Paijens, A. Vledder, M. de Bruyn, H.W. Nijman, "Tumor-infiltrating lymphocytes in the immunotherapy era" Cell. Mol. Immunol., vol. 18, pp. 842-859, 2021. [Crossref]
[7] S. Hendry, R. Salgado, T. Gevaert, P.A. Russell, T. John, B. Thapa, et al., "Assessing tumor infiltrating lymphocytes in solid tumors: A practical review for pathologists and proposal for a standardized method from the International Immuno-oncology Biomarkers Working Group: Part 2: TILs in melanoma, gastrointestinal tract carcinomas, non-small cell lung carcinoma and mesothelioma, endometrial and ovarian carcinomas, squamous cell carcinoma of the head and neck, genitourinary carcinomas, and primary brain tumors" Adv. Anat. Pathol., vol. 24, pp. 311-335, 2017. [PubMed]
[8] A.T. Ruffin, H. Li, L. Vujanovic, D.P. Zandberg, R.L. Ferris, T.C. Bruno, "Improving head and neck cancer therapies by immunomodulation of the tumour microenvironment" Nat. Rev. Cancer, vol. 23, pp. 173-188, 2023. [Crossref]
[9] A. Kumar, R. Watkins, A.E. Vilgelm, "Cell Therapy with TILs: Training and Taming T Cells to Fight Cancer" Front. Immunol., vol. 12, 2021. [Crossref]
[10] M.V. Kiselevskiy, R.Y. Vlasenko, T.N. Zabotina, Z.G. Kadagidze, "Prognostic significance of tumor-infiltrating lymphocytes" Immunologiya, vol. 40, pp. 74-83, 2019. [Crossref]
[11] K. Starska-Kowarska, "The Role of Different Immunocompetent Cell Populations in the Pathogenesis of Head and Neck Cancer—Regulatory Mechanisms of Pro- and Anti-Cancer Activity and Their Impact on Immunotherapy" Cancers, vol. 15, 2023. [Crossref] [PubMed]
[12] I. Plesca, A. Tunger, L. Müller, R. Wehner, X. Lai, M.-O. Grimm, et al., "Characteristics of Tumor-Infiltrating Lymphocytes Prior to and During Immune Checkpoint Inhibitor Therapy" Front. Immunol., vol. 4, 2020. [Crossref]
[13] A. De Meulenaere, T. Vermassen, S. Aspeslagh, K. Vandecasteele, S. Rottey, L. Ferdinande, "TILs in Head and Neck Cancer: Ready for Clinical Implementation and Why (Not)?" Head Neck Pathol., vol. 11, pp. 354-363, 2017. [Crossref]
[14] G.T. Wolf, J.L. Hudson, K.A. Peterson, H.L. Miller, K.D. McClatchey, "Lymphocyte subpopulations infiltrating squamous carcinomas of the head and neck: Correlations with extent of tumor and prognosis" Otolaryngol. Head Neck Surg., vol. 95, pp. 142-152, 1986. [Crossref] [PubMed]
[15] S. Ledderose, H. Schulz, T. Paul, C. Ledderose, G.J. Ledderose, "Characterization of the tumor-infiltrating lymphocyte landscape in sinonasal mucosal melanoma" Pathol. Res. Pract., vol. 241, p. 154289, 2023. [Crossref]
[16] M. Ferrari, L. Alessandrini, E. Savietto, D. Cazzador, G. Schiavo, S. Taboni, et al., "The Prognostic Role of the Immune Microenvironment in Sinonasal Intestinal-Type Adenocarcinoma: A Computer-Assisted Image Analysis of CD3+ and CD8+ Tumor-Infiltrating Lymphocytes" J. Pers. Med., vol. 13, 2023. [Crossref]
[17] M. Torri, A. Sandell, A. Al-Samadi, "The prognostic value of tumor-infiltrating lymphocytes in head and neck squamous cell carcinoma: A systematic review and meta-analysis" Biomed. Pharmacother., vol. 180, 2024. [Crossref] [PubMed]
[18] J.N. Kather, M. Suarez-Carmona, P. Charoentong, C.-A. Weis, D. Hirsch, P. Bankhead, et al., "Topography of cancer-associated immune cells in human solid tumors" Elife, vol. 7, p. e36967, 2018. [Crossref]
[19] B. Shang, Y. Liu, S.J. Jiang, Y. Liu, "Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: A systematic review and meta-analysis" Sci. Rep., vol. 5, 2015. [Crossref]
[20] H. Quan, L. Yan, S. Wang, S. Wang, "Clinical relevance and significance of programmed death-ligand 1 expression, tumor-infiltrating lymphocytes, and p16 status in sinonasal squamous cell carcinoma" Cancer Manag. Res., vol. 11, pp. 4335-4345, 2019. [Crossref]
[21] G. Yin, W. Guo, H. Liu, Z. Huang, X. Chen, "Characteristics of tumor infiltrating lymphocytes in sinonasal mucosal melanoma and prognosis for patients" Curr. Probl. Cancer, vol. 46, p. 100878, 2022. [Crossref] [PubMed]
[22] Y. Liu, N. Zhang, Y. Wen, J. Wen, "Head and neck cancer: Pathogenesis and targeted therapy" MedComm, vol. 5, p. e702, 2024. [Crossref] [PubMed]
[23] S. Pinto, J. Pahl, A. Schottelius, P.J. Carter, J. Koch, "Reimagining antibody-dependent cellular cytotoxicity in cancer: The potential of natural killer cell engagers" Trends Immunol., vol. 43, pp. 932-946, 2022. [Crossref]
[24] N. Kondoh, M. Mizuno-Kamiya, "The Role of Immune Modulatory Cytokines in the Tumor Microenvironments of Head and Neck Squamous Cell Carcinomas" Cancers, vol. 14, 2022. [Crossref]
[25] M. Norouzian, F. Mehdipour, M.J. Ashraf, B. Khademi, A. Ghaderi, "Regulatory and effector T cell subsets in tumor-draining lymph nodes of patients with squamous cell carcinoma of head and neck" BMC Immunol., vol. 23, 2022. [Crossref]
[26] F. Castro, A.P. Cardoso, R.M. Gonçalves, K. Serre, M.J. Oliveira, "Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion" Front. Immunol., vol. 9, 2018. [Crossref] [PubMed]
[27] C. Guillerey, "NK Cells in the Tumor Microenvironment" Adv. Exp. Med. Biol., vol. 1273, pp. 69-90, 2020. [Crossref]
[28] S. Liu, R. Wang, J. Fang, "Exploring the frontiers: Tumor immune microenvironment and immunotherapy in head and neck squamous cell carcinoma" Discov. Oncol., vol. 15, p. 22, 2024. [Crossref]
[29] S. Zöphel, N. Küchler, J. Jansky, C. Hoxha, G. Schäfer, J.J. Weise, "CD16+ as predictive marker for early relapse in aggressive B-NHL/DLBCL patients" Mol. Cancer, vol. 23, p. 210, 2024. [Crossref]
[30] A. Lalos, O. Neri, C. Ercan, A. Wilhelm, S. Staubli, A. Posabella, et al., "High Density of CD16+ Tumor-Infiltrating Immune Cells in Recurrent Ovarian Cancer Is Associated with Enhanced Responsiveness to Chemotherapy and Prolonged Overall Survival" Cancers, vol. 13, 2021. [Crossref]
[31] L. Chen, Y. Chao, W. Li, Z. Wu, Q. Wang, "Soluble immune checkpoint molecules in cancer risk, outcomes prediction, and therapeutic applications" Biomark. Res., vol. 12, 2024. [Crossref] [PubMed]
[32] X. He, C. Xu, "Immune checkpoint signaling and cancer immunotherapy" Cell Res., vol. 30, pp. 660-669, 2020. [Crossref] [PubMed]
[33] K.S. Saini, S. Somara, H.C. Ko, P. Thatai, A. Quintana, Z.D. Wallen, et al., "Biomarkers in head and neck squamous cell carcinoma: Unraveling the path to precision immunotherapy" Front. Oncol., vol. 14, 2024. [Crossref] [PubMed]
[34] Y. Jiang, Y. Li, B. Zhu, "T-cell exhaustion in the tumor microenvironment" Cell Death Dis., vol. 6, pp. e1792-2, 2015. [Crossref]
[35] A. Sharpe, K. Pauken, "The diverse functions of the PD1 inhibitory pathway" Nat. Rev. Immunol., vol. 18, pp. 153-167, 2018. [Crossref]
We use cookies to improve your experience on our site. By continuing to use our site, you accept our use of cookies. Learn more