
APA Style
Nivas Manohar Desai. (2024). Nanotechnology Based Drug Delivery Systems of Herbal Medicine. Biomaterials Connect, 2 (Article ID: 0009). https://doi.org/10.69709/BIOMATC.2024.101090MLA Style
Nivas Manohar Desai. "Nanotechnology Based Drug Delivery Systems of Herbal Medicine". Biomaterials Connect, vol. 2, 2024, Article ID: 0009, https://doi.org/10.69709/BIOMATC.2024.101090.Chicago Style
Nivas Manohar Desai. 2024. "Nanotechnology Based Drug Delivery Systems of Herbal Medicine." Biomaterials Connect 2 (2024): 0009. https://doi.org/10.69709/BIOMATC.2024.101090.
 ACCESS
Review Article
ACCESS
Review Article
Volume 2, Article ID: 2024.0009

Nivas Manohar Desai
nivasdesai88@gmail.com
1 Department of Botany, Dapoli Urban Bank Senior Science College, Dapoli, MS 415712, India
Received: 30 Sep 2024 Accepted: 12 Dec 2024 Available Online: 14 Dec 2024 Published: 20 Dec 2024
Herbal medicine has been utilized extensively throughout ancient times. Advancements in the fields of phytochemistry and phytopharmacology has made it feasible to better understand the chemical composition and biological functions of certain medicinal plant products. The presence of active compounds is necessary for the efficacy of many species of medicinal plants. Most physiologically active components in extracts, such as tannins, terpenoids, and flavonoids, are highly soluble in aqueous solutions but exhibit poor absorption due to their large molecular size, low permeability, or inability to traverse lipid cell membranes. As a result, their bioavailability and efficacy are reduced, making certain extracts unsuitable for healthcare applications. There has been a lot of research into combining nanotechnology with herbal medicine because of the potential for nanostructured systems to enhance the benefits of plant extracts by reducing dosage requirements, minimizing side effects, and enhancing activity. Nanosystems may deliver a sufficient quantity of the active component to the targeted site of action during the course of the treatment. Conventional therapy fails to meet these requirements. The aim of this study is to review nanotechnology-based medicine delivery systems and herbal remedies.
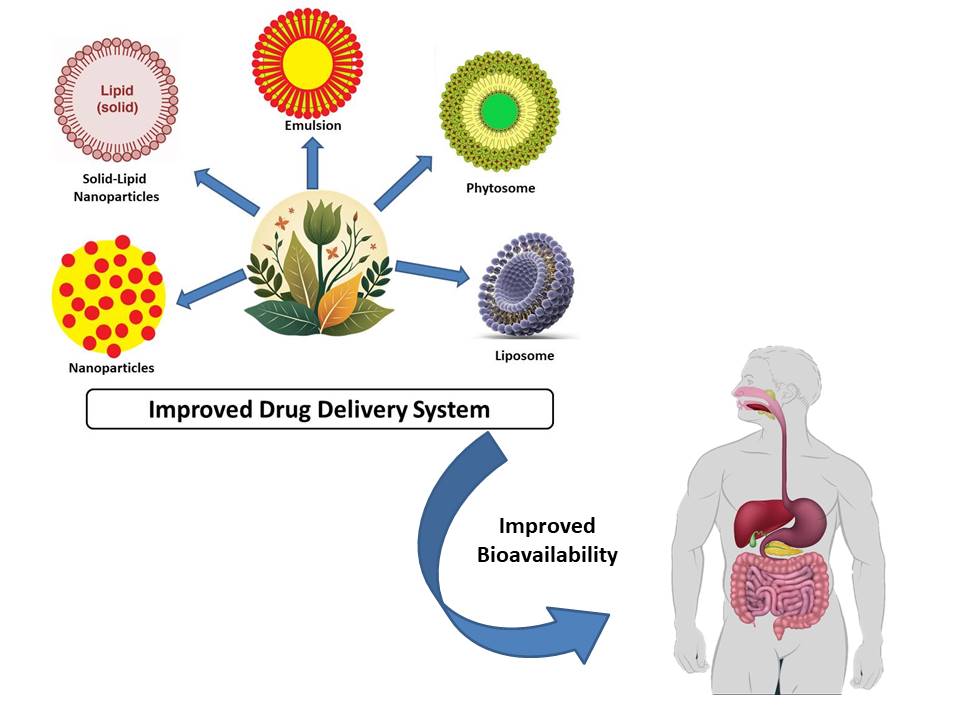
Throughout human evolution, various groups have recognized and utilized plants as herbal medicines. This has been done from the earliest days of human history, when people learned to choose plants for food and medicine. Throughout the second half of the 20th century, however, allopathic medicines gradually replaced herbal therapies, especially in the West. These days, allopathic medicine is more prevalent than traditional medicine, especially in wealthier countries. However, most developing countries continue to use these herbal medicines, likely due to the high cost of synthetic medications [1]. As per the World Health Organization [2], 80 percent of individuals in developing countries depend on traditional medicine to either supplement or fulfill their basic medical requirements. Despite the pharmaceutical industry’s encouragement and support during the creation of allopathic pharmaceuticals, a considerable portion of the population in many countries still seeks medical attention through alternative methods. Many of these rituals have their roots in medicinal herbs. The majority of those who use these natural resources do so because they cannot afford alternative therapies. However, due to global economic, political, and social changes, their therapeutic application has decreased significantly [3,4]. Research on the molecular structure of medicinal plants and their widely used applications is currently a top priority for all scientific associations. The research’s findings may lead to more creative drugs that have fewer negative effects than those that are presently on the market [5]. Natural products’ enormous diversity of physicochemical and biological properties, in addition to their architecture, have also impressed researchers. Still, except for those used for local medicinal requirements, very few plants have been investigated for their potential as medicines. Consequently, there is inadequate data to describe the true potential [6,7,8]. The biological properties of therapeutic plants found all over the world have been studied by numerous research teams. These studies explore the potential benefits of various plant species for the pharmaceutical industry, based on scientific research and public knowledge about the therapeutic benefits of medicinal plants. Approximately 50% of approved medications between 1981 and 2006 were derived, directly or indirectly, from natural ingredients [1]. The chemical intricacy of extracts is crucial to the effectiveness of the formulation, as it needs to release the active ingredient. Therefore, medicines must control both biological response and active absorption while simultaneously boosting drug solubility, delaying drug breakdown, reducing toxicity, and flavor masking [9,10,11]. Through phytochemical and phytopharmacological investigations, the biological characteristics and composition of some medicinal plant products have already been obtained. For the most part, the biologically active components of extracts, like tannins, terpenoids, and flavonoids, are highly water-soluble but have low absorption because they are large, cannot cross lipid membranes, and have poor absorption, which reduces bioavailability and efficacy. Some research indicates that in vitro evaluations of herbal treatments demonstrate great efficacy, which, however, is not consistent with the results of in vivo experiments. Additionally, some critical ingredients are hardly ever employed because they have undesirable qualities or are incompatible with other formulation ingredients [12,13]. Numerous nanotechnological techniques, such as liposomes, polymeric nanoparticles, solid lipid nanoparticles (SLNs), liquid crystal (LC) systems, precursor systems for liquid crystals (PSLCs), and microemulsions, have been tried to break through this obstacle. These tactics have the ability to change an element’s actions within a biological environment and allow the use of many chemicals in the same formulation. These technological developments have changed the way drugs are distributed. The capacity to increase the efficacy of active compounds and restore inert substances that were removed from the formulation, is one of the innovative drug delivery technologies. The potential to enhance novel compounds prior to their therapeutic or commercial application—for example, by increasing their efficacy and selectivity, averting thermal or photodegradation, reducing adverse effects, and controlling the release of active ingredients—further enhances the appeal of this strategy [14,15,16]. Combined with the recent advances in pharmacological research, there is an urgent need for progress in nanoscience and nanotechnology, relevant to the application of nanoscale materials, which had hitherto only been the purview of the cosmetics sector. Solutions to challenging areas of formulation preparation can be enhanced and revolutionized by scientific breakthroughs [17]. Nanostructures can effectively extend the duration of a formulation’s action and mix active substances with differing degrees of lipophilicity and hydrophilicity. Additionally, they can increase the stability and solubility of active ingredients. Additionally, this technology can be used to target the administration of a drug to certain organs or tissues at specific ages [18,19,20,21]. Due to its advantages, such as improved release methods and the ability to design novel formulations that were previously unfeasible (because of numerous variables surrounding the active ingredients), nanotechnology has attracted increasing interest from the pharmaceutical sector [22]. It is important to draw attention to some of the drawbacks of nanotechnology, even though it has benefits for many medical fields. Clinical researchers have identified certain drawbacks, including high expense, challenges in scaling up operations, and the easily inhaled nature of nanoparticles. These particles can cause hazardous lung conditions and often lead to other illnesses that may disrupt homeostasis or even result in death [23,24]. The application of nanotechnology to plant extracts has received an extensive amount of attention in the literature because nanostructured systems have the potential to improve the therapeutic properties of plant extracts. These systems can support the continuous release of active ingredients, require smaller dosage, minimize side effects, and enhance overall activity [25,26]. In a review published by [10], many studies that employed nanostructured devices to improve the properties of botanical extracts were highlighted. In order to increase the absorption of the active components, Bhattacharya and Ghosh [27] used lipid-based systems in combination with infusions of green tea and ginseng (Panax ginseng CA Meyer) (Araliaceae) in a variety of formulations. Rajendran et al. synthesized nanoparticles through a methanolic extract of Ocimum sanctum L. (Lamiaceae) and reported that the encapsulated extract demonstrated stronger antimicrobial properties than in free-form preparation against Escherichia coli, Bacillus subtilis, Pseudomonas aeruginosa, and Staphylococcus aureus [26]. The effectiveness of herbal products, or medicinal plants, depends on the presence of active compounds. Since conventional therapies fall short of these conditions, novel carriers should route the active constituent toward the chosen target and deliver it at a suitable concentration throughout the duration of the therapy. It may be discovered that a certain action is lost entirely or partially when extracted substances are separated or purified. Furthermore, a few constituents are extremely vulnerable to the stomach’s acidic pH, which encourages their breakdown and loss of intended impact after consumption. These barriers prevent the use of some extracts in clinical settings [12]. Numerous nanotechnology-based drug delivery systems, including liposomes, polymeric nanoparticles, solid lipid nanoparticles (SLNs), liquid crystal (LC) systems, precursor systems for liquid crystals (PSLCs), and microemulsions, can be used to improve a formulation’s most desired properties [10]. Furthermore, tiny particles could offer a future where function is assured, overcoming the challenges associated with the use of therapeutic plants. [12].
The field of medication delivery systems with nanostructures is becoming more and more well-known. According to published research, these carriers have great promise for changing the way that phytoactive-based medication delivery methods are used today [29]. Instead, a variety of studies have been conducted and reported on detailing the alteration of the medications’ physicochemical and biological properties. Figure 1 shows the broad application of the nanostructured systems developed for the delivery of phytomedicine. Enhancing the water solubility of weakly soluble phytoactives, such andrographolide and curcumin, has enabled recent advancements in the delivery of phytomedicine [30], as well as the release of herbal substances after oral administration; increase targetability by limiting non-specific medication availability to the undesirable site; increase safety; and enhance biological absorption through the gastrointestinal system [31], simplify the dosage schedule for frequently prescribed drugs; increase the phytoactives’ stability during the extraction, formulation, and storage processes; and improve therapeutic efficacy to increase patient adherence and decrease dose-dependent adverse effects [32,33]. Nanocarriers that facilitate the transfer of phytomolecules to the site of action demonstrate a range of advantages, including phytosomes, nanoparticles, nanocapsules, lipid carriers, and solid lipid nanoparticles [34]. Systems such as this are being developed to treat untreated medical disorders for which traditional dosage forms are not appropriate. By enhancing dissolution, pharmacokinetic properties profile Absorption, distribution, metabolism, and excretion (ADME), accessibility, targetability, effectiveness, and safety, nano-phytomedicines present significant potential for improving the delivery of isolated and validated herbal compounds, proving to be superior to traditional dosage forms.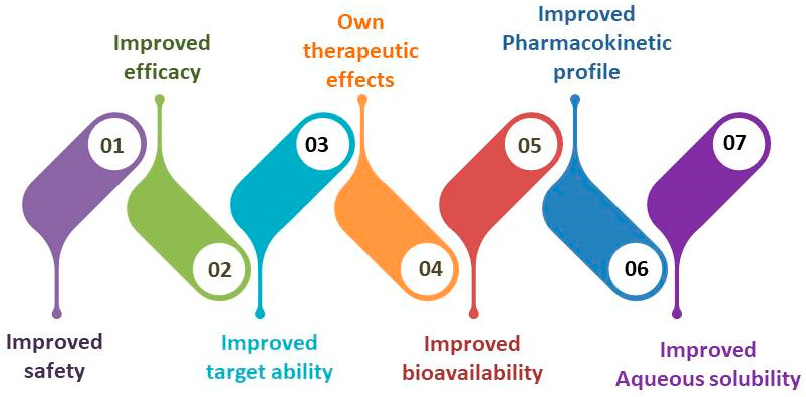
The solubility of phytocompounds is a critical concern that limits their widespread application. Bioactivity-guided fractionation and isolation methods are commonly employed to obtain the majority of phytomolecules. It is not permitted to utilize molecules or extracts other than water-based extracts parenterally or orally. By exercising extra caution while maintaining blood therapeutic levels requires these compounds to be readily accessible in the systemic circulation, the solubility of these molecules inside biological systems has been improved. Reducing the size of the particles, forming solid dispersions and dispersion, adding co-solvents, adding surfactants/solubilizers, etc. are some of the most popular techniques for making the molecules more soluble [35,36]. It has been reported that nanopharmaceuticals improve phytomolecules’ solubility. Due to their capacity to create persistent nanoparticles and droplet that are easily loaded with medications or emulsified to alter their physiochemical properties, nanocarriers are unique techniques for enhancing solubility [37,38]. They have the potential to significantly increase the solubility of phytomolecules; curcumin, for example, has become substantially more soluble when present in nanoparticle form [39]. By transforming BCS class 2 medications into easier to dissolve nano-phytomedicines, oral absorption is improved. Different approaches have been attempted to make these drugs more soluble because this is the rate-limiting phase that keeps a large amount of the medicine out of the systemic circulation (Table 1). The most common methods for achieving solubility augmentation include surface modification with lipids or polymers, nanoparticle formation, and surface area enhancement [39,40]. According to a study by Piazzini et al. [41], Nanoemulsion can improve the permeability of biological membranes and the solubility of Vitex agnus-castus (VAC) extract. Improving oral delivery of VAC extract was the main aim of this approach. The size of the drug-encapsulated nanoparticles was reduced to 11.82~0.125 nm by using triacetin, labrasol, cremophor EL, and water as the oil, surfactant, co-surfactant, and aqueous phase, respectivelywhich resulted in a tenfold increase in VAC solubility. This method made the VAC extract completely soluble in water. Since nanoemulsion has demonstrated high potential in solubilizing the extract, it was expected that improvements in both solubility and permeability would lead to increased oral bioavailability of VAC extract [41]. Systems of nanoparticles have the ability to modify the hydrophobic surface of phytoconstituents. Considerable research has been done on the use of nanoparticles to improve the solubility of hydrophobic medications in water. Nanoparticles are expected to greatly increase the drug’s solubility by dispersing it more broadly. Combining surface modification and particle size reduction has been found to be quite efficient in several cases [42,43]. One approach involves encapsulating two well-known polyphenols, epicatechin and morin, within protein-based nanomaterials using nanoparticles. The combination of morin and epicatechin enhanced the release of drugs in biological systems by incorporating serum albumin nanomaterials having tiny particles molecules (170~6 nm to 200~12 nm) over the surface [40]. It’s not just solubility that matters. It is nearly invariably linked to the dissolution, dispersion, localised availability, and bioavailability of medications. Thus, studies are conducted on it as well as other variables including penetration, absorption, and bioavailability.
Pharmacokinetics describes how the body responds to the absorption, distribution, metabolism, and excretion of an active constituent after administration. The drug goes through the different phases i.e., liberation, absorption, distribution, metabolism and excretion denoted by the term LADME, once it enters the biological milieu of the GIT. These days, the term “liberation” is also being employed. It signifies the release of the active ingredient from the delivery mechanism, which is equally crucial for its absorption. Most pharmacologists believe that the drug’s absorption and release occur during the same procedure. They take into account that the medication is frequently given in an active state, which means that there is no release phase prior to absorption. However, absorption is a pointless phenomenon until the medication is liberated or released from novel carrier systems. Prior to developing the dosage forms’ pharmacokinetic profile, the liberation process needs to be supported by nano-phytomedicine. The term “toxicological aspect” of medicine is frequently shortened to “ADME-Tox” or “ADMET”. Most applications of nano-phytomedicine entail a controlled or gradual release of medicine. When drug release is regulated, the drug remains in the body for a longer amount of time. Increased tissue dispersion and redistribution follow from this, especially when using cytotoxic drugs. As a result, healthy body cells could perish. It is therefore another important issue that is being looked at. The medicine’s ability to effectively cross the biological barrierdepends on a number of factors, such as log P, the mucosal membrane’s thickness, and the availability of the active ingredient in a solubilized state. The drug’s distribution throughout the body and its organs is influenced by a number of parameters, including its biological half-life, pH or temperature stability, and plasma protein binding. Enzymes involved in drug metabolism, such as glucuronosyltransferases and cytochrome P450, can support or impede the chemical breakdown of drugs. The amount of medication that is dispersed throughout the circulatory system and the amount that has been digested determine how much of it is expelled. Different dosages and methods of medicine administration have an impact on pharmacokinetic properties. It is the goal of nano-phytomedicines to alter the pharmacokinetic behaviour of the chosen active ingredients. Encapsulation alters the drug’s surface directly, affecting the rate at which the less absorbed active ingredients are absorbed. Nanoemulsion may be useful in improving solubility, which could raise the permeability of VAC extract when tested using the parallel artificial membrane permeation assay (PAMPA) (Piazzini et al. [41]). When converted into a nanoemulsion, VAC has a higher penetration rate, which may enhance oral bioavailability [41]. The passage of active ingredients through the biological membrane is intimately linked to digestion. Variations in permeability can also occasionally be used to alter the rate of absorption through the skin, whichenables transdermal penetration of the formulation into the skin [44]. According to a study by Lin et al. [45], ultra-deformable lipid (UDL) vesicles are useful for expanding the use of Chinese herbal medicine, especially for the transdermal administration of imperatorin (IMP). When compared to its suspension equivalent, cationic UDLs with smaller particle sizes (>100 nm) and high entrapment efficacy (60.32%~2.82%) increased the amount of IMP that penetrated the skin by 3.45 times. In this instance, modifying the surface of IMP by cationic UDLs proved to be rather advantageous, increasing availability in the various skin layers through enhanced transdermal flux and longer IMP release [45]. The production of a nanoemulsion improved the digestive system’s absorption of phytoestrogenic diarylheptanoids. Gynecological illnesses can be effectively treated with phytoestrogenic diarylheptanoids, which are primarily sourced from C. comosa. Through the development of Other pharmacokinetic requirements, such as distribution, must be satisfied in order to maintain therapeutic blood levels. The drug’s stability at a specific pH or temperature, biological half-life, and reduced plasma protein binding for better dispersion are all improved. Preclinical results by Chang et al. indicate that encapsulating silymarin in liposome and ethosome has much improved its oral bioavailability and tissue distribution. Changes to the liposome and ethosome surface were shown to alter the drug’s pharmacokinetic profile. The same rationale has led to extensive prior research on lipidic and ethanolic encapsulation. Due to the silymarin’s increased solubility in water, it has expanded its distribution within the systemic circulation. Silymarin’s low water solubility was the main factor contributing to its low oral bioavailability; this could be fixed by vesicular techniques, which also improved the drug’s overall therapeutic efficacy [47]. The pharmacokinetics and organ distribution of icariin should be improved by the previously investigated production of PG-liposomes for intraperitoneal administration in small animals. ICAPG-liposomes improved the distribution of icariin into the spleen, liver, lung, kidney, heart, and brain when compared to its solution equivalent. Enhanced AUC(0-t) in the majority of tissues (except from the mouse lung), decreased clearance, extended MRT(0-t), and biological half-life in all tissues (apart from the brain) were the outcomes of the enhanced volume of distribution of icariin in tissues [48]. Li et al.’s most recent study established the potential of liposomal drug delivery technology to improve baicalin’s pharmacokinetics profile and bio-distribution. Chinese hospitals have long used baicalin (BA), which is thought to be the main ingredient in the dry root extract of Scrella baicalensis, in order to treat cerebral ischemia since it is neuroprotective. Its poor lipid solubility severely restricts its oral absorption. To improve its therapeutic relevance, it was developed as a special liposomal carrier (BA-LP). The drug’s lipophilicity was enhanced, leading to an improvement in its level in the brain due to its increased lipophilicity. The particle size range of BA-LP was 160–190 nm, its entrapment efficacy was 42%, and its zeta potential was +5.7 mV. Due to its controlled release features, the drug’s in vitro release kinetic model—which employed the biphasic dynamic model equation—was shown to offer the best fit to BA-LP. There were greater Cmax and AUC0–t, or 1.5–2 times, compared to normal rat brain tissue, whichshows a larger distribution of BA in the brain compared to normal rats. Changes in tissue distribution following liposome production led to decreased amounts of BA in the kidney and higher concentrations in the liver, heart, brain, and lungs. It was believed that BA-LP may serve as a carrier to improve the therapeutic efficacy of molecules similar to BA, as liposomal drug delivery methods were more effective in delivering BA [49]. When it comes to enhancing absorption and pharmacokinetic properties, nanostructured liposomal drug delivery methods have demonstrated significant promise. Plant-based compounds have been extensively researched for their potential to help separate medications from the water phase to the lipid phase, encapsulate aqueous and lipid-based medications, alter the surface, and be compatible with the biological environment. Distribution is essential to drug delivery since it ensures the drugs’ higher bioavailability. Table 1 and Table 2 present an assortment of nanocarriers that are intended to enhance the pharmacokinetic characteristics of pharmaceuticals. Nano-phytomedicines with therapeutic benefits, lipid-based nanomedicines are developed for the enhancement of solubility, pharmacokinetic profile (ADME), bioavailability, target ability, efficacy, and safety. Rai et al. [50]. Source: - Sabuj MZ, Islam N. Nanophytomedicine: An Effective Way for Improving Drug Delivery and Bioavailability of Herbal Medicines. Nanophytomedicine: Concept to Clinic. 2020:55-70 [65]. Nanoemulsions of phytomedicines for the improved solubility, pharmacokinetic profile (ADME), bioavailability, target ability, efficacy, and safety.
Phyto-Constituent
Value Addition
Indication
Route of
AdministrationReference
Liposomes
Silymarin
Improve bioavailability
Hepatoprotective
Buccal
[51]
Artemisia
arborescens essential oilTargeting delivery to cells, enhanced permeation
Antiviral
In vitro
[10]
Garlicin
Increased efficacy
–
–
[12]
Usnea acid
Increased solubility, Improved localization and prolonged release
Anti- mycobacterial
In vitro
[52]
Wogonin
Sustained release effect
Anticancer
In vivo
[53]
Colchicine
Enhance skin deposition, prolonged drug release and improved site specificity
Antigout
Topical
[54]
Catechins
Increased permeation through skin
Antioxidant and chemo-preventive
Transdermal
[55]
Breviscapin
Sustained drug delivery
Cardiovascular diseases
Intramuscular
[56]
Nano-structured lipid/polymeric carriers
Triptolide
Enhanced permeation through stratum corneum by virtue of increased hydration
Anti-inflammatory
Topical
[57]
Flavonoids and lignans
Improved aqueous solubility
Hepatoprotective and antioxidant effects
Oral
[58]
Triptolide
Improved safety
Anti-inflammatory
Oral
[59]
Artemisinin
Sustained drug release
Anticancer
In vitro
[60]
Breviscapine
Prolong the half-life and decreased RES uptake
Cardiovascular and cerebrovascular
Intravenous
[61]
Camptothecin
Prolonged blood circulation and high deposition in tumors
Anticancer
In vitro
[62]
Phytosomes
Silybin Flavonoids
Increased oral absorption of silybin
Hepatoprotective, antioxidant
Oral
[63]
Naringenin
Prolonged duration of action
Antioxidant activity
Oral
[64]
Phyto-Constituent
Value Addition
Indication
Route of
AdministrationReference
Nanoemulsions
Zedoary turmeric oil
Improved aqueous dispersibility, stability and oral bioavailability
Hepatoprotection anticancer and antibacterial
Oral
[66]
Triptolide
Enhanced penetration of drugs through the stratum corneum by increased hydration
Anti-inflammatory
Topical
[67]
Chlorogenic acid from green coffee
Improved aqueous dispersibility, stability and oral bioavailability
Antioxidant activity
Oral
[67]
Salicylic acid
Controlled release and improved dermal retention
Anti-inflammatory activity
Topical
[32]
Mentha essential oil
Control the release, avoid the contact with the skin and reduce the evaporation
Anti-fungal activity against vaginal candidiasis
Topical
[33]
Vesicular systems
Colchicine
Increase skin penetration
Antigout
In vitro
[68]
Matrine
Improved the percutaneous permeation
Anti-inflammatory
Topical
[68]
Microspheres
Zedoary oil
Sustained release and Higher bioavailability
Hepatoprotective
Oral
[69]
Quercetin
Significantly decreases the dose size
Anticancer
In vitro
[70]
Mentha spicata
L. var. viridis oil (MVO)Alleviate skin irritation and volatility and to avoid direct contact with the skin
Anti-fungal activity
Topical
[30]
Reports detail the numerous efforts undertaken by researchers to create nanocarrier systems that improve the solubility and stability of herbal medications. Drug dissolution at blood pH, drug absorption, drug distribution into systemic circulation, first-pass metabolism, GI stability, and drug molecular weight are other factors that affect bioavailability. Part of the phytomedicine efforts to improve bioavailability include increasing medication absorption [71]. Through the development of nanoformulations, silymarin solubility was increased in one study. This improved the drug’s bioavailability and, as a result, its therapeutic efficacy as an antiviral, anticancer, antioxidant, and hepatoprotectant. Water-insoluble active plant components can now be encased in a variety of nanocarriers due to technological advancements in solubility enhancement. The most cutting-edge technology accessible is the creation of nanovesicles and nanocarriers. For example, silymarin may significantly promote passive diffusion in aqueous nanovesicles [65]. Additionally, as cyclovirobuxine D’s solubility improved, SNEDDSs were created to boost the drug’s bioavailability. Propylene glycol, oleic acid, Solutol SH15, and cyclovirobuxine D were combined in the following ratios to create the final Self-Nanoemulsifying Drug Delivery System (SNEDDS) formulation: 3:24:38:38. Scattering of the ultimate SNEDDS structure developed impulsively and was changed into a 64.80 ± 3.58 nm globule-sized nanoemulsion. Greater drug solubility, a quick rate of absorption, an enhanced area under the curve, better penetration, and a decreased efflux were all linked to the investigated medication’s increased dispersion. All of these qualities led to a 200.22% improvement in SNEDDS’s relative bioavailability over commercial tablets. Proposing SNEDDS carriers as a viable option for an oral administration of cyclovirobuxine D-like medications with enhanced bioavailability, the authors highlighted the possible application of SNEDDS in phytomedicine [72]. One of the most researched carriers for increasing the bioavailability of medications is nanoemulsion. Nanoemulsions, colloidal particle systems comprising an organic phase and a water phase, are formulated using an appropriate stabilizing surfactant. These systems are thermodynamically metastable and isotropic in nature. The surfactant-based interface, which can reversibly change the biological membrane’s structure, facilitates drug absorption into the membrane. Its ability to load drugs that are both hydrophilic and hydrophobic is by far its biggest advantage. The various types of nanoemulsions that have been created for various uses are listed in Table 2. Despite being highly soluble in water, breviscapine has a very limited oral bioavailability and this is because to the GIT’s low stability and penetration. By employing nanoemulsion, a nanocarrier that facilitates oral absorption, breviscapine’s bioavailability through the oral route has been increased. It also helped to boost the internal stability of medication. Higher drug encapsulation and constant droplet size contributed to the identification of these features, and it was discovered that the increase in bioavailability might reach 249.7% when compared to relative bioavailability. The reason behind this was the capacity of breviscapine to be encapsulated in a nanoemulsion, so preventing direct exposure to the gastrointestinal environment [73].
When a minority cell or group of minority cells is identified as requiring therapy, it is referred to as a target. For the greatest potential treatment result, enhanced target specificity is meant to aid in pharmacological target identification and maximal drug concentration. In order to prevent pharmaceutically active chemicals, active ingredients, or treatments from spreading to nearby tissues and organs, specific kinds of drug carrier systems are used to deliver them only to the intended site of action. These are delivery or transport vectors that sequester the medication while delivering it to the intended cells. Specifically, targeted areas of action may include the capillary bed, an intracellular region, or a certain cell type. Target ability requires the creation of a carrier with an agent that can selectively recognize a certain target. For instance, the carrier needs to be able to distinguish between normal and tumour cells. When a drug is delivered to its intended location by a carrier, ligands assist in its identification and ensure the carrier is site-specific. Endogenous hormones, polypeptides, and particular antibodies are examples of ligand systems. Treating or preventing diseases is the main way that pharmaceuticals’ target capacities are improved. Biological instability, pharmacokinetic/pharmacodynamic, short biological lifespan, large volume of dispersion, low specificity, low therapeutic index, low drug dissolution, reduced absorption through the gastrointestinal tract, high-plasma adhering of proteins, and instabilities of the drugs in the gastric environment are all taken into account when developing targeted drug delivery systems. Reducing side effects related to the diffusion of drugs into other tissues is the main goal of this strategy. Among other qualities, a system for the delivery of targeted drugs should be immunogenicity-free, chemically and physiologically stable, able to limit drug distribution to specific tissues, cells, or organs, and have a consistent capillary distribution. It has to be under control in addition to manufacturing the drug at the optimal therapeutic dose. A carrier’s design ensures that the least quantity of medication leaks during transit. When designing carriers, additional important considerations include the biodegradability and ease of removal of polymers from the body. These setups should be easy to make, inexpensive, and replicable. When constructing these carriers, other factors are taken into account, such as target cell features, marker traits, or transport carriers—vehicles that deliver medications to specific receptors by using ligands and physically changed components. Cyclovirobotuxine D (CVB-D) is a steroidal alkaloid that is extracted from the Chinese herb Buxus microphylla. It is commonly used to treat cerebrovascular issues and various cardiovascular disorders. The clinical application needs to be enhanced because there aren’t many formulations of CVB-D under investigation. Another drug that may be a bioactive phytoconstituent in triple-negative breast cancer is thymoquinone, which is extracted from the volatile oil of Nigella black cumin seeds. Several molecular targets in cancer, such as ROS, PPAR-γ, p53, p73 STST3, nuclear factor-̡B, and others, are said to exhibit its antineoplastic action [74]. Angiopep-2-conjugated liposomes have shown promise as targeted carriers for brain distribution due to their natural affinity for the low-density lipoprotein receptor-related protein-1 (LRP1), enabling them to cross the blood-brain barrier effectively. There is currently interest in the nasal route of administration due to its ability to target particular brain regions [75]. Furthermore, targeting the liver has shown a lot of promise for nano-phytomedicine [76]. Despite tremendous efforts, millions of individuals worldwide suffer from liver illnesses. Having said this, treating illnesses linked to the liver effectively is immensely imperative. A number of substances, including alcohol, drugs, plants, waterborne and industrial pollutants, and industrial pollutants, can harm the liver and aggravate liver illnesses. Nevertheless, a variety of herbal medicines are used to cure illnesses like cirrhosis, hepatitis, and cancer. Using herbal remedies to treat liver issues is now well established. Nonetheless, the idea of customized drug delivery systems was investigated, ensuring the greatest potential usage of these medications. In liver targeting, receptor ligand binding is the basic concept. There are multiple types of liver-resident receptors on the surface of hepatocytes, including Kupffer cells, hepatic stellate cells, sinusoidal endothelial cells, etc., and they are utilized in an effort to increase the drug’s liver-available form. These receptor targets determine the choice of several ligand types, such as galactosylated, lactobionic acid, and asialofetuin. The ligands are attached to the surface of numerous microparticles, liposomes, nanoparticles, and many more. There have been several particle techniques employed for liver targeting, including liposomes, niosomes, nanoparticles, micelles, and nanosuspensions [77]. Apart from improving the drug’s liver-targeting properties, vinegar-baked Radix Bupleuri (VBRB), a traditional Chinese medication, offers other medical effects as well. When combined with 10-hydroxycamptothecin (HCPT), VBRB’s ability to improve target ability was investigated after loading in polymeric micelles. VBRB dramatically improved the liver-targeting effectiveness of HCPT encapsulated in polymeric micelles when administered in conjunction with oral medicine. Using meridian directed medications in concert with modern drug delivery methods to better target active pharmaceuticals was a simple, yet effective strategy that was demonstrated by this approach [78]. According to a review by Bartneck et al. (2014), nanomedicine may be used to treat liver fibrosis and inflammation [79]. It is possible that some nanomaterials will trigger macrophage activation, such as AuNRs (gold nanorods treated with peptides). Targeting mannose receptors can be utilized to guide macrophages in liver diseases. Targeting HSC, which are responsible for collagen production, is achievable using nanoconstructs designed to identify specific markers such as insulin-like growth factor II (IGF-II), platelet-derived growth factor (PDGF) receptor β, peroxisome proliferator-activated receptor 1 (PPAR1), and integrins that are active during fibrosis. [79]. A polymeric nanoparticle-based intraperitoneal injection of curcumin called NanoCurcTM has been developed. This technique successfully reduced liver damage and fibrosis [80]. Quercetin is a subcutaneously injected nanomedicine that binds to the galactosyl receptor preferentially and efficiently to hepatocytes [81]. Consequently, the utilization of macromolecular carriers emerged as a feasible approach for efficient bone targeting [82]. To prevent steroid-related osteonecrosis in small animals, icaritin has been administered via a new bone-targeted drug delivery technique. This approach has been shown to be effective in avoiding steroid-induced osteonecrosis. By encouraging bone formation and decreasing fat deposition and bone resorption, it works at osteoonecrotic responsive skeletal locations. Once the impact of a novel phytomolecule, icaritin, on osteogenesis was discovered, the authors developed a bone-targeted delivery method with anti-bone resorption and anti-adipogenesis effects. Both Asp8-liposome and liposome-icaritin have not shown any discernible effectiveness against high SAON. However, in rats treated with steroids, Asp8-liposome-icaritin significantly decreased the death of osteocytes, downregulated osteoclastogenesis, and upregulated osteogenesis. Administering Asp8-liposome-icaritin resulted in the prevention of bone resorption, suppression of adiposeness, and enhancement of bone formation [83]. Nanomedicine has been applied to hair follicles in addition to the liver, kidneys, and cancers. Bendable liposomes that are resistant to methicillin-resistant Staphylococcus aureus (MRSA) enhanced hair follicle targeting and made it possible to administer chloramphenicol for more efficient therapy of difficult-to-treat bacterial invasions of hair follicles. Deformable liposomes were discovered to have good biocompatibility against keratinocytes and neutrophils and to be safe for topical application on skin [84]. These findings highlight the great potential of nanomedicine in reaching any level of the body, i.e., cells, tissues, or organs, with phytoconstituent delivery. These characteristics of nanomedicine are caused by the nanocarriers’ versatile shape and structure, ultra-deformable integrity, surface charge, nano size range, and variety of polymer properties. The potential for targeting phytoconstituents to the brain, liver, kidney, bone, tumor site, and specific cells is considerable; nevertheless, additional study is required to optimize their therapeutic pertinence and incorporate these carriers into a highly scalable production method.
Physicochemical aspects of the drugs also demonstrate an increase in safety. A potential avenue for enhancing safety is the study of nanophytomedicine and safe medication distribution, which is supported by a ton of new research. Improved safety is a further application for nanomedicine, and these carriers are quite helpful in handling such situations. The purpose of the thymoquinone-rich fraction nanoemulsion (TQRFNE) was to increase the acute toxicity of thymoquinone. TQRFNE lessened the drug’s associated toxicity in experiments conducted on Sprague Dawley rats. Comparing the TQRFNE group to the group that received distilled water, it was discovered that characteristics such common behavior, body weight, food and water consumption, relative organ weight, hematological, histology, and clinical biochemistry remained unaltered. Furthermore, throughout the experimental period, no reports of fatalities or toxicological symbols were recorded [85]. Using Multispectral Optoacoustic Tomography (MSOT) imaging, the potency of a conjugated polymer-based ratiometric nanoprobe against in vivo herb-induced liver injury has been evaluated. This ratiometric nanoprobe was designed for in vivo imaging of hepatic injury employing MSOT imaging, which enables precise identification of the site of liver injury. In order to create an internal standard, the liposomal nanoprobe was made up of a responsive dye (IX-2NH2) that could only react with NO and a conjugated polymer based on diketopyrrolopyrrole (DPP-TT). Using 3D data from the mouse model, ratiometric optoacoustic diagnosis of liver damage caused by herbal medicines offers a method to reduce liver toxicity without invasive procedures [86]. These processes have been developed into multiple variations to date. Nanomedicine-based molecular imaging has been acknowledged as a significant field in safety pharmacology. Optoacoustic and photoacoustic imaging, CT scanning, Magnetic resonance imagings (MRIs), ultrasounds, and scanning are among the techniques now being employed to improve patient safety.
Proteins, bioactive lipids, and miRNA are examples of extracellular messengers that function as cell-to-cell messengers in a manner similar to the exosomes secreted by mammalian cells [87]. When it comes to getting low dose drugs (such proteins, peptides, and siRNAs) to where they are supposed to go, chemically manufactured nanoparticles have two main disadvantages. Before the created nanoparticles are deemed therapeutically useful, their components need to be assessed for low fabrication scalability and probable in vivo safety. However, plant-based nanoparticles are thought to be able to get beyond these restrictions. It is noteworthy that ingesting these nanoparticles has no risks. In interspecies communication, they may serve as natural cures against certain ailments. Moreover, nanoparticles based on plant-derived lipids can help precisely transport drugs to their targeted site. Vegetable nanoparticles derived from plants could be easily manufactured on a big scale, unlike synthetically created nanoparticles. The grape juice nanocarriers, ginger, carrot juice, and tomato juice are extracted using eco-friendly methods. In the field of nanomedicine, these nano-phytocomposites offer a fresh approach to producing therapeutic nanoparticles [88]. Nanoparticles made of botanical ingredients are vital for interspecies communication and beneficial against inflammatory diseases and cancer. Recent findings indicate that oral administration of grape exosome-based nanocarriers to mice promotes the growth of intestinal stem cells throughout the intestine and intestinal epithelial cell proliferation. Moreover, it seemed that saliva, digestive fluids, and intestinal proteolytic enzymes could not degrade grape exosome-like oral nanoparticles. Based on the currently available information, ingestible plant-based nanoparticles can enter the colon through the oral route, where they can be absorbed by intestinal cells and utilized for various purposes like intestinal regeneration. Edible nanoparticles with anti-inflammatory and colonic tissue-targeting properties could be a novel, nontoxic delivery method for the management of gastrointestinal tract pain, including inflammatory bowel disease, and could be readily developed on a large scale [89]. Metallic nanoparticles can have additional or beneficial effects when used in combination with other medicinal treatments, and they are also used as a medicinal ingredient. Among the metallic nanoparticles that have been produced are Ag, Au, Fe, and others. When ingested, the chemical process utilized to produce metallic nanoparticles is hazardous and deadly. As a result, “green” synthesis methods in chemistry are gaining traction. These regulations are desperately needed in light of the environmental problems. It has been demonstrated to have strong bactericidal and inhibitory properties in addition to anti-fungal, anti-inflammatory, and anti-angiogenesis effects. These attributes make it valuable in the disciplines of catalysis, biosensors, high sensitivity biomolecular recognition, and medicine. Many methods, such as ion sputtering, chemical reduction, and sol gel, can be used to create silver nanoparticles [90]. The production of silver nanoparticles using a unique reducing agent was summarized by Khan et al. This “green reductant” was made from plant extracts and used as a reducing agent to create silver nanoparticles. This investigation was carried out in response to the increased demand for silver nanoparticles (Ag-NPs) in the pharmaceutical industry. These nanoparticles are used in the biomedical sector because of their antibacterial, antifungal, or anticancer properties. The usage of hazardous chemicals in the chemical synthesis process makes alternative methods necessary, even if it is an effective way to produce nanoparticles with the necessary size, shape, and crystallinity. Nevertheless, the resulting Ag-NPs’ size, shape, and crystallinity are not under the control of these biological processes. Novel bio-inspired synthetic procedures have been investigated and have demonstrated some promise in reducing adverse environmental effects [91,92]. An overview of Ag-NP manufacturing via chemical and environmentally friendly methods is given in their compilation. “Green synthesis” was developed as a response to the limitations of chemical and biological synthesis. In order to create Ag-NPs with the right size, morphology, shape, crystallinity, and biological effect while using the fewest number of hazardous materials possible, various plant extracts and phytomolecules were used, along with their chemical and biological characteristics. Their action on particle synthesis was demonstrated. Olive, beet root, mangosteen, Ziziphora tenuior, Abutilon indica, Solanum tricobatum, Erythrina indica, leaf extracts of It has been documented that silver nanoparticles (silver-NPs) range in size from 5 to 20 nm. The potential biological applications of silver nanoparticles surface functionalized by phytomolecules are now under investigation [93]. Ag-NPs’ antibacterial properties have led to their extensive use in the medical field, textile coatings, food preservation, wound healing, color reduction, and other environmental applications [94,95]. Compounds containing elemental silver have shown potentialin treating a range of bacterial and fungal illnesses. Using A. indica extract-mediated silver nanoparticles, potential antibacterial agents have been investigated. Silver nanoparticles produced by green synthesis have been shown to have higher antibacterial action against
When a drug-loaded innovative carrier system is created using nanotechnology-based methods, it is commonly referred to as nanomedicine. This is because the systems can be effectively used for disease prevention, diagnostics, or therapy. The careful integration of nanotechnology concepts and methods with phytomedicines has become a significant priority in recent times. Researchers are becoming increasingly interested in using these formulations to treat and control chronic diseases like cancer, arthritis, such as, Parkinson’s, Alzheimer’s, and epilepsy, and other conditions because of their success and potential at the clinical level. These formulations’ improved absorption and bioavailability, rapid dissolution, quicker onset of action, higher plasma concentration, prolonged actions, site-specific targeting, decreased toxicity, and improved pharmacological performances have already increased interest in these formulations. In summary, the pharmaceutical industries are drawn to the integration of new technology in the production of herbal medicine-based preparations, as it offers superior alternatives to conventional dosage forms, hence improving people’s health through the use of these “herbal remedies”. Even though nanotechnology offers immense potential and benefits, only a small number of these products—nanophytomedicinal meagre—have found their way into clinical settings. This is because such products require sophisticated machinery, complicated scaling procedures, high preparation costs, potential health and environmental risks from produced nanoparticles, and short shelf lives for developed formulations. These issues remain significant obstacles that must be addressed in a very systematic manner.
SLNs
Solid Lipid Nanoparticles
LC
Liquid Crystal
PSLCs
Precursor Systems for Liquid Crystals
ADME
Absorption, Distribution, Metabolism, and Excretion
VAC
Vitex agnus-castus
GIT
Gastrointestinal Tract
DME-Tox
Absorption, Distribution, Metabolism, Excretion, and Toxicity
LADME
Liberation, Absorption, Distribution, Metabolism and Excretion
PAMPA
Parallel Artificial Membrane Permeation Assay
UDL
Ultra-Deformable Lipid
BA
Baicalin
BA-LP
Baicalin Liposomal Carrier
SNEDDS
Self-Nanoemulsifying Drug Delivery System
CVB-D
Cyclovirobotuxine D
LRP1
Lipoprotein Receptor-Related Protein-1
VBRB
Vinegar-Baked Radix Bupleuri
HCPT
Hydroxycamptothecin
IGF-II
Insulin-Like Growth Factor II
PDGF
Platelet-Derived Growth Factor
MRSA
Methicillin-Resistant Staphylococcus Aureus
TQRFNE
Thymoquinone-Rich Fraction Nanoemulsion
MSOT
Multispectral Optoacoustic Tomography
MRIs
Magnetic Resonance Imagings
Ag-NPs
Silver Nanoparticles
The author is solely responsible for the conception, design, analysis, interpretation, drafting, and final approval of the article.
The author declares no conflicts of interest regarding this manuscript.
The author received no financial support for the manuscript.
[1] T.M. Souza-Moreira, H. Salgado, R.C. Pietro, "O Brasil no contexto de controle de qualidade de plantas medicinais" Rev. Bras. De Farmacogn., vol. 20, pp. 435-440, 2010. [Crossref]
[2] A.M. Pires, P.S. Araújo, "Percepção de risco e conceitos sobre plantas medicinais, fitoterápicos e medicamentos alopáticos entre gestantes" Rev. Baiana De Saúde Pública, vol. 35, p. 320, 2011. [Crossref]
[3] V.F. Ferreira, A.C. Pinto, "A fitoterapia no mundo atual" Química Nova, vol. 33, p. 1829, 2010. [Crossref]
[4] M.R. Badke, M.D. Budó, F.M. Silva, L.B. Ressel, "Plantas medicinais: O saber sustentado na prática do cotidiano popular" Esc Anna Nery, vol. 15, pp. 132-139, 2011. [Crossref]
[5] E.M. Costa, A.S. Barbosa, T.A. Arruda, P.T. Oliveira, F.R. Dametto, R.A. Carvalho, et al., "In vitro antimicrobial activity of plant extracts against Enterococcus faecalis" J. Bras. De Patol. E Med. Lab., vol. 46, pp. 175-180, 2010. [Crossref]
[6] S. Verma, S.P. Singh, "Current and future status of herbal medicines" Vet. World, vol. 1, p. 347, 2008. [Crossref]
[7] T.M.B. Bresolin, V.C. Filho, "," in Fármacos e Medicamentos: Uma Abordagem Multidisciplinar [Drugs and medicines: A Multidisciplinary Approach], , Eds. São Paulo, Brazil: Santos, 2010, . (In Portuguese)
[8] L.P. Mazzolin, A.L. Nasser, T.M. Moraes, R.C. Santos, C.M. Nishijima, F.V. Santos, et al., "Qualea parviflora Mart.: An integrative study to validate the gastroprotective, antidiarrheal, antihemorragic and mutagenic action" J. Ethnopharmacol., vol. 127, pp. 508-514, 2010. [Crossref] [PubMed]
[9] C.E. Kluczynik, J.H. Souza, J.D. Palmeira, S.B. Ferreira, R.M. Antunes, T.A. Arruda, et al., "Perfil de sensibilidade de Salmonella sp. de ambiente aquático a antimicrobianos comerciais e a extratos hidroalcoólicos de plantas medicinais" Rev. Bras. Anal. Clin., vol. 42, pp. 141-144, 2010. Available online: https://pesquisa.bvsalud.org/portal/resource/pt/lil-558435.
[10] K. Kesarwani, R. Gupta, "Bioavailability enhancers of herbal origin: An overview" Asian Pac. J. Trop. Biomed., vol. 3, pp. 253-266, 2013. [Crossref] [PubMed]
[11] K. Holmberg, "," in Handbook of Applied Surface and Colloid Chemistry, , Eds. Hoboken, NJ, USA: John Wiley & Sons, Ltd., 2002, . Available online: https://www.wiley.com/en-us/Handbook+of+Applied+Surface+and+Colloid+Chemistry%2C+2+Volume+Set-p-9780471490838.
[12] S.S. Ajazuddin, "Applications of novel drug delivery system for herbal formulations" Fitoterapia, vol. 81, pp. 680-689, 2010. [Crossref] [PubMed]
[13] R.M. Mainardes, M.C. Urban, P.O. Cinto, M.V. Chaud, R.C. Evangelista, M.D. Gremiao, "Liposomes and micro/nanoparticles as colloidal carriers for nasal drug delivery" Curr. Drug Deliv., vol. 3, p. 275, 2006. [Crossref]
[14] A.E. Grill, N.W. Johnston, T. Sadhukha, J. Panyam, "A review of select recent patents on novel nanocarriers" Recent Pat. Drug Deliv. Formul., vol. 3, pp. 137-142, 2009. [Crossref]
[15] J. Venugopal, M.P. Prabhakaran, S. Low, A.T. Choon, G. Deepika, V.R. Giri Dev, et al., "Continuous nanostructures for the controlled release of drugs" Curr. Pharm. Des., vol. 15, pp. 1799-1808, 2009. [Crossref] [PubMed]
[16] M. Chorilli, A.C. Brizante, C.A. Rodrigues, H.R. Salgado, E. Pombo-Nascimento, D.M. Ventura, "Aspectos gerais em sistemas transdérmicos de liberação de fármacos" Rev. Bras. Farm., vol. 88, pp. 7-13, 2007.
[17] M.M. Baley, C.J. Berkland, "Nanoparticle formulations in pulmonar drug delivery" Med. Res. Rev., vol. 29, pp. 196-212, 2009. [Crossref]
[18] Y. Chen, X. Lin, H. Park, R. Greever, "Study of artemisinin nanocapsules as anticancer drug delivery systems" Nanomed. Nanotechnol. Biol. Med., vol. 5, pp. 316-322, 2009. [Crossref]
[19] K.C. Pestana, T.P. Formariz, C.M. Franzini, V.H. Sarmento, L.A. Chiavacci, M.V. Scarpa, et al., "Oil-in-water lecithin-based microemulsions as a potential delivery system for amphotericin, B" Colloids Surf. B Biointerfaces, vol. 66, pp. 253-259, 2008. [Crossref]
[20] A.D. Cunha Júnior, S.L. Fialho, L.B. Carneiro, F. Oréfice, "Microemulsões como veículo de drogas para administração ocular tópica" Arq. Bras. De Oftalmol., vol. 66, pp. 385-391, 2003. [Crossref]
[21] A.C. Sintov, L. Shapiro, "New microemulsion vehicle facilitates percutaneous penetration in vitro and cutaneous drug bioavailability in vivo" J. Control. Release, vol. 95, pp. 173-183, 2004. [Crossref]
[22] R.W. Lee, D.B. Shenoy, R. Sheel, "Micellar nanoparticles: Applications for topical and passive transdermal drug delivery," in Handbook of Non-Invasive Drug Delivery Systems, , Eds. Norwich, NY, USA: William Andrew Publishing, 2010, pp. 37-58.
[23] A. Yadav, M. Ghune, D.K. Jain, "Nano-medicine based drug delivery system" J. Adv. Pharm. Educ. Res., vol. 1, pp. 201-213, 2011. Available online: https://japer.in/article/nano-medicine-based-drug-delivery-system.
[24] R. Singh, S. Tiwari, J. Tawaniya, "Review on nanotechnology with several aspects" Int. J. Res. Comput. Eng. Electron., vol. 2, pp. 1-8, 2013. Available online: https://paper.researchbib.com/view/paper/3824.
[25] V. Ghosh, S. Saranya, A. Mukherjee, N. Chandrasekaran, "Antibacterial microemulsion prevents sepsis and triggers healing of wound in wistar rats" Colloids Surf. B Biointerfaces, vol. 105, pp. 152-157, 2013. [Crossref] [PubMed]
[26] R. Rajendran, R. Radhai, T.M. Kotresh, E. Csiszar, "Development of antimicrobial cotton fabrics using herb loaded nanoparticles" Carbohydr. Polym., vol. 91, pp. 613-617, 2013. [Crossref] [PubMed]
[27] S. Bhattacharya, A.K. Ghosh, "Phytosomes: The emerging technology for enhancement of bioavailability of botanicals and nutraceuticals" Int. J. Aesthetic Antiaging Med., vol. 2, pp. 87-91, 2009.
[28] Y.L. Su, Z.Y. Fu, J.Y. Zhang, W.M. Wang, H. Wang, Y.C. Wang, "Preparation of Radix salvia nanoparticles" Powder Technol., vol. 184, pp. 114-121, 2008. [Crossref]
[29] V.K. Rai, N. Mishra, K.S. Yadav, N.P. Yadav, "Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: Formulation development, stability issues, basic considerations and applications" J. Control. Release, vol. 270, pp. 203-225, 2018. [Crossref]
[30] N. Mishra, K.S. Yadav, V.K. Rai, N.P. Yadav, "Polysaccharide encrusted multilayered nano-colloidal system of andrographolide for improved hepatoprotection" AAPS PharmSciTech, vol. 18, pp. 381-392, 2017. [Crossref] [PubMed]
[31] N. Mishra, V.K. Rai, K.S. Yadav, P. Sinha, A. Kanaujia, D. Chanda, et al., "Encapsulation of mentha oil in chitosan polymer matrix alleviates skin irritation" AAPs PharmSciTech, vol. 17, pp. 482-492, 2016. [Crossref]
[32] P. Sinha, N. Srivastava, V.K. Rai, R. Mishra, P.V. Ajayakumar, N.P. Yadav, "A novel approach for dermal controlled release of salicylic acid for improved anti-inflammatory action: Combination of hydrophilic-lipophilic balance and response surface methodology" J. Drug Deliv. Sci. Technol., vol. 52, pp. 870-884, 2019. [Crossref]
[33] N. Srivastava, D.K. Patel, V.K. Rai, A. Pal, N.P. Yadav, "Development of emulgel formulation for vaginal candidiasis: Pharmaceutical characterization, in vitro and in vivo evaluation" J. Drug Deliv. Sci. Technol., vol. 48, pp. 490-498, 2018. [Crossref]
[34] A. Sharma, D.K. Upadhyay, G.S. Sarma, N. Kaur, G.D. Gupta, R.K. Narang, et al., "Squalene integrated NLC based gel of tamoxifen citrate for efficient treatment of psoriasis: A preclinical investigation" J. Drug Deliv. Sci. Technol., vol. 56, p. 101568, 2020. [Crossref]
[35] B. Kumar, M. Pandey, F.H. Pottoo, F. Fayaz, A. Sharma, P.K. Sahoo, "Liposomes: Novel drug delivery approach for targeting Parkinson’s disease" Curr. Pharm. Des., vol. 26, pp. 4721-4737, 2020. [Crossref]
[36] S. Sharma, S.A. Rabbani, J.K. Narang, F.H. Pottoo, J. Ali, S. Kumar, et al., "Role of rutin nanoemulsion in ameliorating oxidative stress: Pharmacokinetic and pharmacodynamics studies" Chem. Phys. Lipids, vol. 228, p. 104890, 2020. [Crossref] [PubMed]
[37] M.S. Alam, M.N. Javed, F.H. Pottoo, A. Waziri, F.A. Almalki, M.S. Hasnain, et al., "QbD approached comparison of reaction mechanism in microwave synthesized gold nanoparticles and their superior catalytic role against hazardous nirto-dye" Appl. Organomet. Chem., vol. 33, p. e5071, 2019. [Crossref]
[38] M.N. Javed, M.S. Alam, A. Waziri, F.H. Pottoo, A.K. Yadav, M.S. Hasnain, et al., "QbD applications for the development of nanopharmaceutical products," in Pharmaceutical Quality by Design, , Eds. Cambridge, MA, USA: Academic Press, 2019, pp. 229-253.
[39] R.K. Bhawana Basniwal, H.S. Buttar, V.K. Jain, N. Jain, "Curcumin nanoparticles: Preparation, characterization, and antimicrobial study" J. Agric. Food Chem., vol. 59, pp. 2056-2061, 2011. [Crossref] [PubMed]
[40] P. Ghosh, S. Bag, A.S. Roy, E. Subramani, K. Chaudhury, S. Dasgupta, "Solubility enhancement of morin and epicatechin through encapsulation in an albumin based nanoparticulate system and their anticancer activity against the MDA-MB-468 breast cancer cell line" RSC Adv., vol. 6, pp. 101415-29, 2016. [Crossref]
[41] V. Piazzini, E. Monteforte, C. Luceri, E. Bigagli, A.R. Bilia, M.C. Bergonzi, "Nanoemulsion for improving solubility and permeability of Vitex agnus-castus extract: Formulation and in vitro evaluation using PAMPA and Caco-2 approaches" Drug Deliv., vol. 24, pp. 380-390, 2017. [Crossref]
[42] M.A. Ansari, I.M. Chung, G. Rajakumar, M.A. Alzohairy, M.N. Alomary, M. Thiruvengadam, et al., "Current nanoparticle approaches in nose to brain drug delivery and anticancer therapy—A review" Curr. Pharm. Des., vol. 26, pp. 1128-1137, 2020. [Crossref]
[43] M.A. Ansari, K.F. Badrealam, A. Alam, S. Tufail, G. Khalique, M.J. Equbal, et al., "Recent nano-based therapeutic intervention of bioactive sesquiterpenes: Prospects in cancer therapeutics" Curr. Pharm. Des., vol. 26, pp. 1138-1144, 2020. [Crossref]
[44] N. Ahmad, R. Ahmad, A. Al-Qudaihi, S.E. Alaseel, I.Z. Fita, M.S. Khalid, et al., "Preparation of a novel curcumin nanoemulsion by ultrasonication and its comparative effects in wound healing and the treatment of inflammation" RSC Adv., vol. 9, pp. 20192-20206, 2019. [Crossref] [PubMed]
[45] H. Lin, Q. Xie, X. Huang, J. Ban, B. Wang, X. Wei, et al., "Increased skin permeation efficiency of imperatorin via charged ultradeformable lipid vesicles for transdermal delivery" Int. J. Nanomed., vol. 8, pp. 831-842, 2018. [Crossref] [PubMed]
[46] J. Su, K. Sripanidkulchai, Y. Hu, R. Chaiittianan, B. Sripanidkulchai, "Increased in situ intestinal absorption of phytoestrogenic diarylheptanoids from Curcuma comosa in nanoemulsions" AAPS PharmSciTech, vol. 14, pp. 1055-1062, 2013. [Crossref]
[47] L.W. Chang, M.L. Hou, T.H. Tsai, "Silymarin in liposomes and ethosomes: Pharmacokinetics and tissue distribution in free-moving rats by high-performance liquid chromatography–tandem mass spectrometry" J. Agric. Food Chem., vol. 62, pp. 11657-11665, 2014. [Crossref] [PubMed]
[48] W. Yang, X.C. Yu, X.Y. Chen, L. Zhang, C.T. Lu, Y.Z. Zhao, "Pharmacokinetics and tissue distribution profile of icariin propylene glycol-liposome intraperitoneal injection in mice" J. Pharm. Pharmacol., vol. 64, pp. 190-198, 2012. [Crossref]
[49] N. Li, L. Feng, Y. Tan, Y. Xiang, R. Zhang, M. Yang, "Preparation, characterization, pharmacokinetics and biodistribution of baicalin-loaded liposome on cerebral ischemia-reperfusion after iv administration in rats" Molecules, vol. 23, 2018. [Crossref] [PubMed]
[50] V.K. Rai, G.D. Gupta, F.H. Pottoo, M.A. Barkat, "Potential of Nano-Structured Drug Delivery System for Phytomedicine Delivery," in Nanophytomedicine, S. Beg and M. Barkat and F. Ahmad, Eds. Singapore: Springer, 2020, .
[51] M.S. El-Samaligy, N.N. Afifi, E.A. Mahmoud, "Increasing bioavailability of silymarin using a buccal liposomal delivery system: Preparation and experimental design investigation" Int. J. Pharm., vol. 308, pp. 140-148, 2006. [Crossref] [PubMed]
[52] E.A. Mendonça, M.C. Lira, M.M. Rabello, I.M. Cavalcanti, S.L. Galdino, I.R. Pitta, et al., "Enhanced antiproliferative activity of the new anticancer candidate LPSF/AC04 in cyclodextrin inclusion complexes encapsulated into liposomes" AAPS PharmSciTech, vol. 13, pp. 1355-1366, 2012. [Crossref] [PubMed]
[53] A. Mosaddik, V. Ravinayagam, S. Elaanthikkal, H. Fessi, W. Badri, A. Elaissari, "Development and use of polymeric nanoparticles for the encapsulation and administration of plant extracts," in Natural Products as Source of Molecules with Therapeutic Potential: Research & Development, Challenges and Perspectives, , Eds. Berlin/Heidelberg, Germany: Springer, 2018, pp. 391-463.
[54] A.K. Barupal, V. Gupta, S. Ramteke, "Preparation and characterization of ethosomes for topical delivery of aceclofenac" Indian J. Pharm. Sci., vol. 72, p. 582, 2010. [Crossref]
[55] N.M. Morsi, A.A. Aboelwafa, M.H. Dawoud, "Improved bioavailability of timolol maleate via transdermal transfersomal gel: Statistical optimization, characterization, and pharmacokinetic assessment" J. Adv. Res., vol. 7, pp. 691-701, 2016. [Crossref]
[56] H. Zhong, Y. Deng, X. Wang, B. Yang, "Multivesicular liposome formulation for the sustained delivery of breviscapine" Int. J. Pharm., vol. 301, pp. 15-24, 2005. [Crossref]
[57] Z. Mei, H. Chen, T. Weng, Y. Yang, X. Yang, "Solid lipid nanoparticle and microemulsion for topical delivery of triptolide" Eur. J. Pharm. Biopharm., vol. 56, pp. 189-196, 2003. [Crossref] [PubMed]
[58] F.L. Yen, T.H. Wu, L.T. Lin, T.M. Cham, C.C. Lin, "Nanoparticles formulation of Cuscuta chinensis prevents acetaminophen-induced hepatotoxicity in rats" Food Chem. Toxicol., vol. 46, pp. 1771-1777, 2008. [Crossref] [PubMed]
[59] S. Jain, S. Jain, P. Khare, A. Gulbake, D. Bansal, S.K. Jain, "Design and development of solid lipid nanoparticles for topical delivery of an anti-fungal agent" Drug Deliv., vol. 17, pp. 443-451, 2010. [Crossref]
[60] P. Kothamasu, H. Kanumur, N. Ravur, C. Maddu, R. Parasuramrajam, S. Thangavel, "Nanocapsules: The weapons for novel drug delivery systems" BioImpacts BI, vol. 2, 2012. [Crossref]
[61] M. Liu, H. Li, G. Luo, Q. Liu, Y. Wang, "Pharmacokinetics and biodistribution of surface modification polymeric nanoparticles" Arch. Pharmacal Res., vol. 31, pp. 547-554, 2008. [Crossref]
[62] K.H. Min, K. Park, Y.S. Kim, S.M. Bae, S. Lee, H.G. Jo, et al., "Hydrophobically modified glycol chitosan nanoparticles-encapsulated camptothecin enhance the drug stability and tumor targeting in cancer therapy" J. Control. Release, vol. 127, pp. 208-218, 2008. [Crossref] [PubMed]
[63] X. Yanyu, S. Yunmei, C. Zhipeng, P. Qineng, "The preparation of silybin–phospholipid complex and the study on its pharmacokinetics in rats" Int. J. Pharm., vol. 307, pp. 77-82, 2006. [Crossref] [PubMed]
[64] K. Maiti, K. Mukherjee, V. Murugan, B.P. Saha, P.K. Mukherjee, "Exploring the effect of hesperetin–HSPC complex—A novel drug delivery system on the in vitro release, therapeutic efficacy and pharmacokinetics" AAPS PharmSciTech, vol. 10, pp. 943-950, 2009. [Crossref]
[65] M.Z. Sabuj, N. Islam, "Nanophytomedicine: An Effective Way for Improving Drug Delivery and Bioavailability of Herbal Medicines," in Nanophytomedicine: Concept to Clinic, , Eds. Berlin/Heidelberg, Germany: Springer, 2020, pp. 55-70.
[66] Y. Zhao, C. Wang, A.H. Chow, K. Ren, T. Gong, Z. Zhang, et al., "Self-nanoemulsifying drug delivery system (SNEDDS) for oral delivery of Zedoary essential oil: Formulation and bioavailability studies" Int. J. Pharm., vol. 383, pp. 170-177, 2010. [Crossref]
[67] N.M. Desai, J. Gilbert Stanley, P.S. Murthy, "Green coffee nanoparticles: Optimisation, in vitro bioactivity and bio-release property" J. Microencapsul., vol. 37, pp. 52-64, 2020. [Crossref] [PubMed]
[68] Y. Zheng, S.X. Hou, T. Chen, Y. Lu, "Preparation and characterization of transfersomes of three drugs in vitro" Zhongguo Zhong Yao Za Zhi= Zhongguo Zhongyao Zazhi= China J. Chin. Mater. Medica, vol. 31, pp. 728-731, 2006. [PubMed]
[69] J. You, X. Han, Y.S. Wang, L. Yang, Y.W. Yu, Q.P. Li, "Study of the preparation of sustained-release microspheres containing zedoary turmeric oil by the emulsion–solvent-diffusion method and evaluation of the self-emulsification and bioavailability of the oil" Colloids Surf. B Biointerfaces, vol. 48, pp. 35-41, 2006. [Crossref] [PubMed]
[70] P. Chao, M. Deshmukh, H.L. Kutscher, D. Gao, S.S. Rajan, P. Hu, et al., "Pulmonary targeting microparticulate camptothecin delivery system: Anticancer evaluation in a rat orthotopic lung cancer model" Anti-Cancer Drugs, vol. 21, pp. 65-76, 2010. [Crossref]
[71] A. Di Costanzo, R. Angelico, "Formulation strategies for enhancing the bioavailability of silymarin: The state of the art" Molecules, vol. 24, 2019. [Crossref]
[72] Z. Ke, X. Hou, X.B. Jia, "Design and optimization of self-nanoemulsifying drug delivery systems for improved bioavailability of cyclovirobuxine, D" Drug Des. Dev. Ther., vol. 28, pp. 2049-2060, 2016. [Crossref] [PubMed]
[73] Y. Ma, H. Li, S. Guan, "Enhancement of the oral bioavailability of breviscapine by nanoemulsions drug delivery system" Drug Dev. Ind. Pharm., vol. 41, pp. 177-182, 2015. [Crossref]
[74] M.A. Barkat, J. Ahmad, M.A. Khan, S. Beg, F.J. Ahmad, "Insights into the targeting potential of thymoquinone for therapeutic intervention against triple-negative breast cancer" Curr. Drug Targets, vol. 19, pp. 70-80, 2018. [Crossref] [PubMed]
[75] H. Wei, T. Liu, N. Jiang, K. Zhou, K. Yang, W. Ning, et al., "A novel delivery system of cyclovirobuxine D for brain targeting: Angiopep-conjugated polysorbate 80-coated liposomes via intranasal administration" J. Biomed. Nanotechnol., vol. 14, pp. 1252-1262, 2018. [Crossref] [PubMed]
[76] M.A. Harshita Barkat, M. Rizwanullah, S. Beg, F.H. Pottoo, S. Siddiqui, F.J. Ahmad, "Paclitaxel-loaded nanolipidic carriers with improved oral bioavailability and anticancer activity against human liver carcinoma" AAPS PharmSciTech, vol. 20, p. 87, 2019. [Crossref] [PubMed]
[77] R. Rohilla, T. Garg, A.K. Goyal, G. Rath, "Herbal and polymeric approaches for liver-targeting drug delivery: Novel strategies and their significance" Drug Deliv., vol. 23, pp. 1645-1661, 2016. [Crossref] [PubMed]
[78] H. Wu, T. Yu, Y. Tian, Y. Wang, R. Zhao, S. Mao, "Enhanced liver-targeting via coadministration of 10-Hydroxycamptothecin polymeric micelles with vinegar baked Radix Bupleuri" Phytomedicine, vol. 44, pp. 1-8, 2018. [Crossref]
[79] M. Bartneck, K.T. Warzecha, F. Tacke, "Therapeutic targeting of liver inflammation and fibrosis by nanomedicine" Hepatobil. Surg. Nutr., vol. 3, p. 364, 2014. [Crossref]
[80] S. Bisht, M.A. Khan, M. Bekhit, H. Bai, T. Cornish, M. Mizuma, et al., "A polymeric nanoparticle formulation of curcumin (NanoCurc™) ameliorates CCl4-induced hepatic injury and fibrosis through reduction of pro-inflammatory cytokines and stellate cell activation" Lab. Investig., vol. 91, pp. 1383-1395, 2011. [Crossref] [PubMed]
[81] A.K. Mandal, S. Das, M.K. Basu, R.N. Chakrabarti, N. Das, "Hepatoprotective activity of liposomal flavonoid against arsenite-induced liver fibrosis" J. Pharmacol. Exp. Ther., vol. 320, pp. 994-1001, 2007. [Crossref] [PubMed]
[82] S. Yamashita, H. Katsumi, T. Sakane, A. Yamamoto, "Bone-targeting dendrimer for the delivery of methotrexate and treatment of bone metastasis" J. Drug Target., vol. 26, pp. 818-828, 2018. [Crossref]
[83] S. Chen, L. Zheng, J. Zhang, H. Wu, N. Wang, W. Tong, et al., "A novel bone targeting delivery system carrying phytomolecule icaritin for prevention of steroid-associated osteonecrosis in rats" Bone, vol. 106, pp. 52-60, 2018. [Crossref]
[84] C.Y. Hsu, S.C. Yang, C.T. Sung, Y.H. Weng, J.Y. Fang, "Anti-MRSA malleable liposomes carrying chloramphenicol for ameliorating hair follicle targeting" Int. J. Nanomed., vol. 12, pp. 8227-8238, 2017. [Crossref] [PubMed]
[85] Z. Tubesha, M.U. Imam, R. Mahmud, M. Ismail, "Study on the potential toxicity of a thymoquinone-rich fraction nanoemulsion in Sprague Dawley rats" Molecules, vol. 18, pp. 7460-7472, 2013. [Crossref]
[86] Y. Wu, L. Sun, F. Zeng, S. Wu, "A conjugated-polymer-based ratiometric nanoprobe for evaluating in-vivo hepatotoxicity induced by herbal medicine via MSOT imaging" Photoacoustics, vol. 13, pp. 6-17, 2019. [Crossref] [PubMed]
[87] F.H. Pottoo, M.A. Barkat, M.A. Ansari, M.N. Javed, Q.M. Jamal, M.A. Kamal, "Nanotechnological based miRNA intervention in the therapeutic management of neuroblastoma," in Seminars in Cancer Biology, , Eds. Cambridge, MA, USA: Academic Press, 2021, pp. 100-108.
[88] M. Zhang, E. Viennois, C. Xu, D. Merlin, "Plant derived edible nanoparticles as a new therapeutic approach against diseases" Tissue Barriers, vol. 4, p. e1134415, 2016. [Crossref]
[89] S. Ju, J. Mu, T. Dokland, X. Zhuang, Q. Wang, H. Jiang, et al., "Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis" Mol. Ther., vol. 21, pp. 1345-1357, 2013. [Crossref] [PubMed]
[90] S. Ahmed, M. Ahmad, B.L. Swami, S. Ikram, "Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract" J. Radiat. Res. Appl. Sci., vol. 9, pp. 1-7, 2016. [Crossref]
[91] S. Mishra, S. Sharma, M.N. Javed, F.H. Pottoo, M.A. Barkat, M.S. Alam, et al., "Bioinspired nanocomposites: Applications in disease diagnosis and treatment" Pharm. Nanotechnol., vol. 7, pp. 206-219, 2019. [Crossref]
[92] S. Sharma, M.N. Javed, F.H. Pottoo, S.A. Rabbani, M.A. Barkat, M. Sarafroz, et al., "Bioresponse inspired nanomaterials for targeted drug and gene delivery" Pharm. Nanotechnol., vol. 7, pp. 220-233, 2019. [Crossref]
[93] M. Khan, M.R. Shaik, S.F. Adil, S.T. Khan, A. Al-Warthan, M.R. Siddiqui, et al., "Plant extracts as green reductants for the synthesis of silver nanoparticles: Lessons from chemical synthesis" Dalton Trans., vol. 47, pp. 11988-12010, 2018. [Crossref]
[94] Y.A. Qing, L. Cheng, R. Li, G. Liu, Y. Zhang, X. Tang, et al., "Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies" Int. J. Nanomed., vol. 5, pp. 3311-3327, 2018. [Crossref] [PubMed]
[95] M.A. Barkat, S. Beg, M. Naim, F.H. Pottoo, S.P. Singh, F.J. Ahmad, "Current progress in synthesis, characterization and applications of silver nanoparticles: Precepts and prospects" Recent Pat. Anti-Infect. Drug Discov., vol. 13, pp. 53-69, 2018. [Crossref]
Disclaimer: All statements, viewpoints, and data presented in this article are the sole responsibility of the individual author(s) and contributor(s) and do not represent those of their affiliated institutions, the publisher, the editor(s), or reviewers. The publisher and its editor(s) accept no liability for any damage to individuals or property that may result from the use of ideas, methods, instructions, or products discussed within the content.
We use cookies to improve your experience on our site. By continuing to use our site, you accept our use of cookies. Learn more