
APA Style
Asmaa Mohamed. (2025). Azilsartan Kamedoxomil: Solubility Enhancement Techniques. Biomaterials Connect, 2 (Article ID: 0012). https://doi.org/10.69709/BIOMATC.2024.178190MLA Style
Asmaa Mohamed. "Azilsartan Kamedoxomil: Solubility Enhancement Techniques". Biomaterials Connect, vol. 2, 2025, Article ID: 0012, https://doi.org/10.69709/BIOMATC.2024.178190.Chicago Style
Asmaa Mohamed. 2025. "Azilsartan Kamedoxomil: Solubility Enhancement Techniques." Biomaterials Connect 2 (2025): 0012. https://doi.org/10.69709/BIOMATC.2024.178190.
 ACCESS
Mini-Review
ACCESS
Mini-Review
Volume 2, Article ID: 2025.0012

Asmaa Mohamed
asmaa.abdelaziz@alzahraa.edu.iq
1 College of Pharmacy, Al-Zahraa University for Women, Karbala, Iraq
Received: 01 Nov 2024 Accepted: 20 Jan 2025 Available Online: 20 Apr 2025 Published: 07 Mar 2025
Azilsartan kamedoxomil (AZ) belongs to biopharmaceutical solubility class IV (BCS IV) that has low solubility and low permeability. Therefore, several techniques should be utilized to enhance its bioavailability, especially solubility enhancement. The most critical step in achieving the AZ desired strength in systemic circulation for anticipated pharmacological action is the dissolution of AZ. The low solubility of new chemicals is a significant obstacle in formulation and generic development. For AZ to be absorbed, it must exist in a soluble state at the site of action. Diverse approaches are employed to improve its solubility, including physicochemical changing of AZ or other techniques like particle size reduction, solid dispersion, and complexation. Improving AZ solubility depends on drug characteristics, absorption site, and dosage requirements. The recent techniques of solubilization and enhancing bioavailability were the focus of this review.
Azilsartan kamedoxomil is a prodrug quickly hydrolyzed to azilsartan, the active metabolite. It is employed as antihypertensive and can manage mild to moderate cases of essential hypertension [1,2]. A solute’s ability to mix evenly with a solvent is known as its solubility. A substance’s solubility is affected by temperature and pressure [3,4]. The solvent could be a single chemical or a mixture of liquids [5]. Solubility should not be confused with the ability of a material to liquefy [6,7]. Their poor bioavailability challenges the formulation of oral dosage forms. Many factors impact bioavailability, including solubility, permeability, dissolution, and first-pass metabolism, while poor bioavailability is referred to as low solubility and permeability [8,9]. High doses of poorly soluble drugs are often needed to attain therapeutic plasma concentrations after ingestion. The low water solubility of new drugs and generic development is a significant obstacle in formulation. An aqueous solution is required for any drug to be absorbed. Most drugs exhibit weak acidity or basicity, resulting in limited water solubility [4] More than 40% of new drugs invented are practically insoluble in water. Consequently, the drug is absorbed slowly, which leads to poor bioavailability. For orally administered drugs, solubility is the most critical factor in determining the rate at which the desired concentration in the bloodstream is achieved for a pharmacological response [10]. Therefore, improving the solubility and bioavailability of a drug is one of the biggest obstacles in formulation. There are different methods published in the literature to increase the solubility. The selected technique relied on various characteristics of the drug and the desired dosage form [3]. This review discusses in detail the several techniques that could help AZ, a candidate drug of BCS IV, enhance its solubility and bioavailability due to increased permeability.
2.1. Nano Suspension A pharmaceutical nanosuspension consists of nanoparticles stabilized by various surfactants, commonly utilized for poorly soluble drugs. The particle size distribution in nanosuspensions ranges between 200 and 600 nm [11]. Nanosuspensions are the optimized technique in BCS class-II and IV drug formulation since they result in high dissolution [12]. Another advantage of nanosuspension is that it can be easily manufactured [12]. Rajab and Jassem (2017) prepared and characterized AZ nanosuspension using different stabilizers such as polyethylene glycol 6000 (PEG 6000) with other co-stabilizers (Tween8). AZ nanosuspension exhibited rapid dispersibility and in-vitro dissolution [13]. 2.2. Self-Emulsifying Drug Delivery System (SMEDDS) and SMEDDS is an anhydrous system of microemulsions that is comprised of oil, surfactant and cosurfactant. The drug is dissolved in a mixture of surfactant and oil, forming oil-in-water microemulsions. SMEDDS revealed improve physical stability [14,15]. Madan et al. developed AZ-SMEDDS for solubility enhancement of AZ containing Syloid® XDP 3150 had improved in vitro solubility when compared to pure AZ [16] Nanoemulsion was designated a drug delivery system to enhance solubility and permeability, reduce adverse effects, elevate efficacy, and ease production and bioavailability [17,18]. Several variables are critical in developing nanoemulsions and should be optimized [19]. These variables could be optimized by employing the central composite design (CCD) and the full-factorial design [20]. Kumar et al. (2022) developed an AZ nanoemulsion employing CCD to enhance its solubility and permeability using ethyl oleate, tween 80, and Transcutol P [21]. 2.3. Solid Dispersion Solid dispersion makes the drug(s) dispersed in a carrier at a solid state designed by fusion methods [22]. Solid dispersion is the most commonly employed technique for enhancing the solubility of drugs [23]. Many polymers are employed, such as polyvinylpyrrolidone and polyethylene glycol 4000 and 6000. Soluplus® with amphiphilic possessions that have dual character [24,25] This method uses an appropriate preparation technique to thoroughly distribute a medication in a water-soluble carrier. Particle size reduction typically speeds up the process of the drug changing from an amorphous to a crystalline state, which is a highly soluble, high-energy state. Recently, the use of hydrophilic carriers has enhanced the wettability of drug particles. Further, solid medication dispersion aids in reducing drug particle size [26,27]. Das et al. developed AZ solid dispersions based on polyvinylpyrrolidone to enhance solubility. The investigation encompasses utilizing solvent evaporation and kneading techniques for solid dispersion preparation. Results indicate that solid dispersions produced through the solvent evaporation method exhibit superior enhancements in solubility, compared to those prepared via kneading [28]. 2.4. Liquisolid Approach The liquid solid technique could transform liquid or dissolved drugs into free-flowing, compressible powders [29], in which the drug has a large surface area that permits the drug to dissolve freely and achieve excellent wettability [30,31]. Water-miscible organic liquids like propylene glycol and polyethylene glycol 400, are employed. Sometimes, lipid-based liquid vehicles, like Captex, may increase the solubility of the drug when incorporated [32]. Chopra et al. developed AZ liquisolid compacts that have been designed employing Capmul MCM and Captex as lipid vehicles. The formulations were evaluated for in vivo bioavailability, demonstrating a 1.29-fold increase in AZ bioavailability compared to the pure drug. AZ [32]. 2.5. Complexation Diverse complexation strategies employing cyclodextrins (CDs) have acquired good favor in recent years for increasing the solubilization of BCS II and IV. CDs are molecules with a hydrophilic exterior and lipophilic central cavity that locate lipophilic drugs. This inclusion complex formation enhances drug solubility and bioavailability by incorporating the drug molecule into the cavity [33,34]. CDs comprised (α-CD), (β-CD), or (γ-CD) units, having a hydrophilic hydroxyl group on their exterior surface and a hydrophobic hollow inside [35,36,37]. He et al. employed γ-CD metal-organic framework (CD-MOF) large molecular cages in which AZ was located, obtaining nanoclusters. AZ nanocluster solubility was raised 340-fold compared to the AZ [38]. 2.6. Hydrotropy Hydrotrophy is a solubilization approach where the expansion of the second solute, the hydrotropic operator, extends the fluid solvency of the first solute [39]. Adding substances or salts that expand dissolvability [40]. Ultrasonic may improve dissolvability due to complexation via the connection between the hydrotropic operators such as sodium benzoate, sodium acetic acid, sodium alginate, and urea and the drug [41,42,43] Surwade et al. (2015) investigated AZ’s solubility, which was determined separately in some hydrotropic operators at different concentrations, and the highest solubilization was obtained in a 40% sodium benzoate that included in urea: sodium acetate: sodium benzoate in 5:20:15 ratio that was utilized to prepare solid dispersions that released >92% within 45 min [44]. 2.7. Nonocrystals Nanocrystals have sizes ranging from 10 to 1000 nm stabilized by stabilizers [45]. Nanocrystals are manufactured by many procedures, such as milling, high-pressure homogenization, and supercritical fluid technology; He et al. employed machine learning techniques to anticipate nanocrystals’ particle size [46,47]. Per the Noyes–Whitney equation, nanocrystals with extensive surface areas could significantly enhance solubility [48,49]. Ma et al. improved the solubility and in-vivo bioavailability of AZ by the development of nanocrystals utilizing a bead milling method employing sodium deoxycholate with Poloxamer 188 [50].
Fourier-transform infrared (FTIR) Spectrophotometric study employing product spectra were compared to the pure drug where peaks were observed [43]. AZ’s powder X-ray diffraction PXRD and its characteristic peaks were investigated. Differential scanning calorimetry (DSC), and thermogravimetric analysis (TGA) are employed to assess any complex formation or binding. Zeta potential analysis is conducted in the case of microencapsulation. Additionally, solubility and powder dissolution rate analyses are performed [51]. In order to provide a synopsis of the procedures and approaches utilized for AZ solubility and characterization in order to guarantee the enhancement of solubility The approaches of AZ solubility and characterization are illustrated in Figure 1.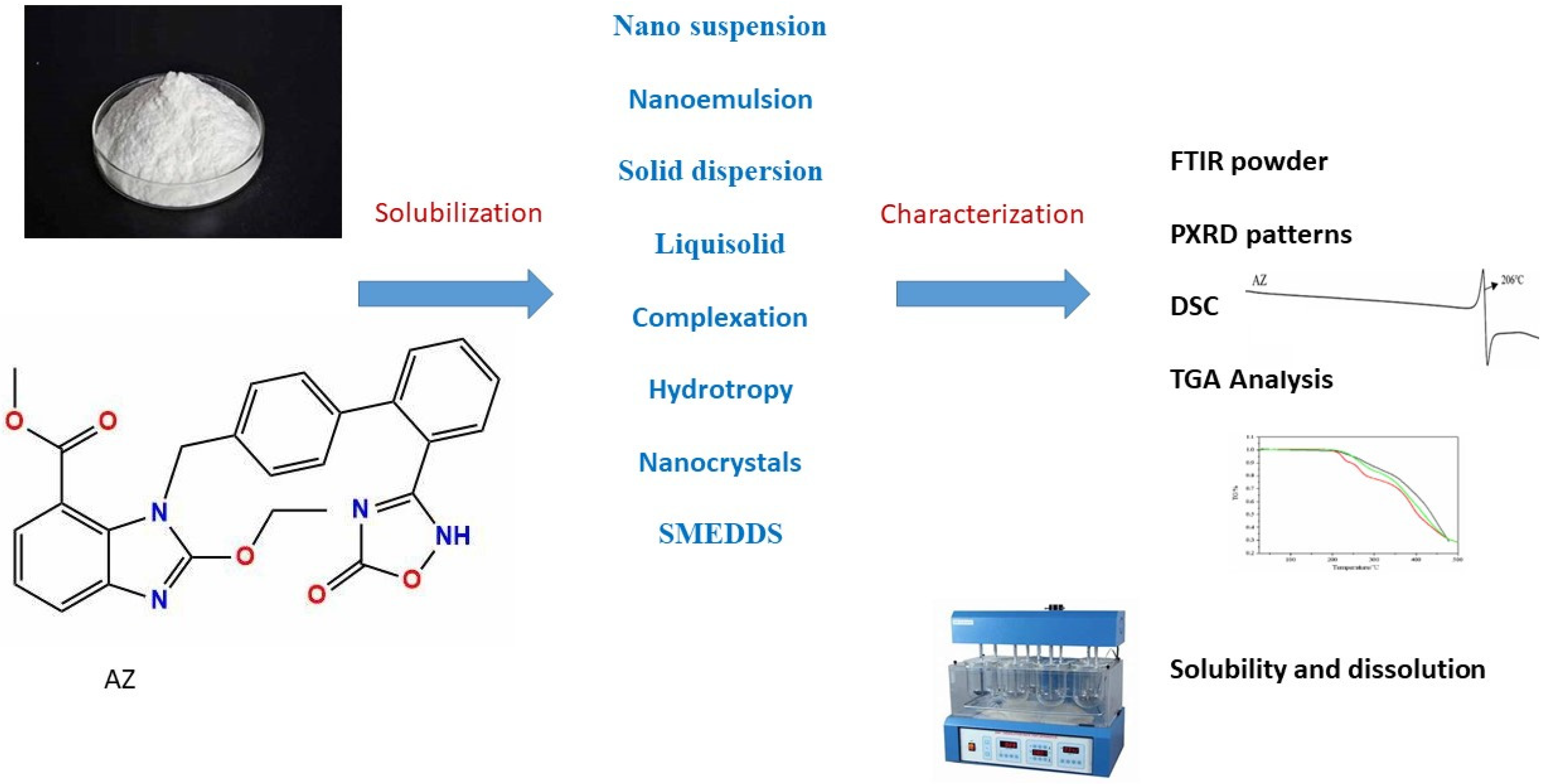
The review discussed many technologies that enable the dissolution of AZ. Moreover, they maximize drug dispersion, thereby improving oral bioavailability. Multiple investigations have revealed rapid dissolution and AZ absorption within formulations. One or more technologies could end the obstacles of low dissolution and absorption of AZ as a BCS IV candidate.
| AZ | Azilsartan Kamedoxomil |
| BCS | Biopharmaceutical Solubility Class |
| CDs | Cyclodextrins |
| PEG 6000 | Polyethylene Glycol 6000 |
| SMEDDS | Self-Emulsifying Drug Delivery System |
| CCD | Central Composite Design |
| FTIR | Fourier-Transform Infrared |
| PXRD | Powder X-Ray Diffraction PXRD |
| DSC | Differential Scanning Calorimetry |
| TGA | Thermogravimetric Analysis |
The author confirms that she is solely responsible for the conception, design, analysis, interpretation, drafting, and final approval of the article.
Not Applicable.
The author declares no conflicts of interest.
This study received no external funding.
I thank Al-Zahraa University for Women for their support.
[1] A. Pradhan, A. Tiwari, R. Sethi, "Azilsartan: Current Evidence and Perspectives in Management of Hypertension" Int. J. Hypertens., vol. 2019, p. 1824621, 2019. [Crossref] [PubMed]
[2] K.T. Savjani, A.K. Gajjar, J.K. Savjani, "Drug solubility: Importance and enhancement techniques" Int. Sch. Res. Not., vol. 2012, p. 195727, 2012. [Crossref]
[3] A. Kumar, S.K. Sahoo, K. Padhee, P.S. Kochar, A. Sathapathy, N. Pathak, "Review on solubility enhancement techniques for hydrophobic drugs" Pharm. Glob., vol. 3, pp. 001-007, 2011. Available online: https://auctoresonline.org/article/review-on-solubility-enhancement-techniques-for-poorly-soluble-drugs.
[4] V.R. Vemula, V. Lagishetty, S. Lingala, "Solubility enhancement techniques" Int. J. Pharm. Sci. Rev. Res., vol. 5, pp. 41-51.4, 2010. Available online: https://globalresearchonline.net/journalcontents/volume5issue1/article-007.pdf.
[5] P. Jain, A. Goel, S. Sharma, M. Parmar, "Solubility enhancement techniques with special emphasis on hydrotrophy" Int. J. Pharma Prof. Res., vol. 1, pp. 34-45, 2010. Available online: https://ijppronline.com/index.php/IJPPR/article/view/104.
[6] A.R. Kale, S. Kakade, A. Bhosale, "A Review on: Solubility Enhancement Techniques" J. Curr. Pharma Res., vol. 10, p. 3630, 2020. Available online: https://jcpr.humanjournals.com/wp-content/uploads/2021/02/4.Anjali-R.-Kale-Sujit-Kakade-Ashok-Bhosale.pdf.
[7] S. Jagtap, C. Magdum, D. Jadge, R. Jagtap, "Solubility enhancement technique: A review" J. Pharm. Sci. Res., vol. 10, pp. 2205-2211, 2018. Available online: https://www.jpsr.pharmainfo.in/Documents/Volumes/vol10Issue09/jpsr10091818.pdf.
[8] Y.S.R. Krishnaiah, "Pharmaceutical technologies for enhancing oral bioavailability of poorly soluble drugs" J. Bioequivalence Bioavailab., vol. 2, pp. 28-36, 2010. [Crossref]
[9] K.H. Edward, D. Li, "," in Drug Like Properties: Concept, Structure, Design and Methods, from ADME to Toxicity Optimization, , Eds. Amsterdam, The Netherlands: Elsevier, 2008, p. 56.
[10] D. Sharma, M. Soni, S. Kumar, G.D. Gupta, "Solubility enhancement—Eminent role in poorly soluble drugs" Res. J. Pharm. Technol., vol. 2, pp. 220-224, 2009. Available online: https://rjptonline.org/HTML_Papers/Research%20Journal%20of%20Pharmacy%20and%20Technology__PID__2009-2-2-46.html#:~:text=Solid%20dispersions%20have%20tremendous%20potential%20for%20improving%20drug,KEYWORDS%3A%20Solid%20dispersion%2C%20solubility%2C%20eutectic%20mixture%2C%20poorly%20soluble.
[11] S.G. Pınar, A.N. Oktay, A.E. Karaküçük, N. Çelebi, "Formulation Strategies of Nanosuspensions for Various Administration Routes" Pharmaceutics, vol. 15, 2023. [Crossref]
[12] R. Pignatello, C. Bucolo, G. Spedalieri, A. Maltese, G. Puglisi, "Flurbiprofen loaded acrylate polymer nanosuspensions for ophthalmic application" Biomater. Sci., vol. 23, pp. 3247-3255, 2002. [Crossref] [PubMed]
[13] N. Ayash, N. Jassem, "Design and in vitro evaluation of azilsartan medoxomil as self-dispersible dry nanosuspension" J. Int. Pharm. Res., vol. 45, pp. 49-68, 2018. [Crossref]
[14] A.S. Deshmukh, "Recent advances in self-emulsifying drug delivery system" Int. J. Pharm. Sci. Nanotechnol., vol. 8, pp. 1-5, 2015. [Crossref]
[15] P. Sriamornsak, S. Limmatvapirat, S. Piriyaprasarth, Z. Mansukmanee P and Huang, "A New Self-Emulsifying Formulation of Mefenamic Acid with Enhanced Drug Dissolution" Asian J. Pharm. Sci., vol. 10, pp. 121-127, 2015. [Crossref]
[16] J.R. Madan, K. Patil, R. Awasthi, K. Dua, "Formulation and evaluation of solid self-microemulsifying drug delivery system for azilsartan medoxomil" Int. J. Polym. Mater. Polym. Biomater., vol. 70, pp. 100-116, 2019. [Crossref]
[17] D.J. McClements, "Advances in Edible Nanoemulsions: Digestion, Bioavailability, and Potential Toxicity" Prog. Lipid Res., vol. 81, p. 101081, 2021. [Crossref] [PubMed]
[18] M. Kumar, R.S. Bishnoi, A.K. Shukla, C.P. Jain, "Techniques for Formulation of Nanoemulsion Drug Delivery System: A Review" Prev. Nutr. Food Sci., vol. 24, pp. 225-234, 2019. [Crossref] [PubMed]
[19] P.R. Amarachinta, G. Sharma, N. Samed, A.K. Chettupalli, M. Alle, J.C. Kim, "Central Composite Design for the Development of Carvedilol-Loaded Transdermal Ethosomal Hydrogel for Extended and Enhanced Anti-Hypertensive Effect" J. Nanobiotechnol. Nol., vol. 19, 2021. [Crossref]
[20] C.L. Ngan, M. Basri, F.F. Lye, H.R. Fard Masoumi, M. Tripathy, R. Abedi Karjiban, et al., "Comparison of Box– Behnken and Central Composite Designs in Optimization of Fullerene Loaded Palm-Based Nano-Emulsions for Cosmeceutical Application" Ind. Crop. Prod., vol. 59, pp. 309-317, 2014. [Crossref]
[21] G. Kumar, T. Virmani, K. Pathak, O. Al Kamaly, A. Saleh, "Central Composite Design Implemented Azilsartan Medoxomil Loaded Nanoemulsion to Improve Its Aqueous Solubility and Intestinal Permeability: In Vitro and Ex Vivo Evaluation" Pharmaceuticals, vol. 15, 2022. [Crossref] [PubMed]
[22] D.B. Deshmukh, P.D. Gaikwad, V.H. Bankar, S.P. Pawar, "Dissolution enhancement of poorly water soluble diacerein by solid dispersion technique" J. Pharm. Sci. Res., vol. 2, p. 734, 2010. Available online: https://www.jpsr.pharmainfo.in/Documents/Volumes/Vol2Issue11/jpsr%2002101109.pdf.
[23] A.N. Patil, D.M. Shinkar, R.B. Saudagar, "Solubility enhancement by solid dispersion" Int. J. Curr. Pharm. Res., vol. 9, pp. 15-18, 2017. [Crossref]
[24] R.N. Shamma, M. Basha, "Soluplus®: A novel polymeric solubilizer for optimization of carvedilol solid dispersions: Formulation design and effect of method of preparation" Powder Technol., vol. 237, pp. 406-414, 2013. [Crossref]
[25] J. Tamboli, S.K. Mohite, "Immediate release solid dispersion tablet of azilsartan: Formulation strategy to enhance oral bioavailability" Int. J. Appl. Pharm., pp. 126-134, 2020. [Crossref]
[26] S. Kumar, R. Agrawal, "Solubility Enhancement of Poorly Water-Soluble Drugs by Solid Dispersion Techniques" Int. J. Pharma Prof. Res., vol. 14, pp. 84-106, 2023. [Crossref]
[27] K. Jadhav, S. Hegaje, S. Jadhav, R. Vaidhya, M. Redkar, "Formulation and Evaluation of Solid Dispersion of Poorly Soluble Drugs" Asian J. Pharm. Technol., vol. 12, pp. 310-312, 2022. [Crossref]
[28] B. Das, A. Patra, K. Julekha, J. Baldota, N. Dutta, M. Malakar, et al., "Development and Characterization of Modified Dosage Forms of Azilsartan Medoxomil for Improved Solubility and Oral Bioavailability" Afr. J. Biol. Sci., vol. 6, pp. 1343-1353, 2024. Available online: https://www.afjbs.com/issue-content/development-and-characterization-of-modified-dosage-forms-of-azilsartan-medoxomil-for-improved-solubility-and-oral-bioavailability-1100.
[29] M.D. Prajapat, S.B. Butani, M.C. Gohel, "Liquisolid: A promising technique to improve dissolution efficiency and bioavailability of poorly water soluble nimodipine" J. Drug Deliv. Sci. Technol., vol. 53, p. 101135, 2019. [Crossref]
[30] P.O. Nnamani, A.A. Ugwu, E.C. Ibezim, F.C. Kenechukwu, P.A. Akpa, J.-D.N. Ogbonna, et al., "Sustained-release liquisolid compact tablets containing artemether-lumefantrine as alternate-day regimen for malaria treatment to improve patient compliance" Int. J. Nanomed., vol. 11, pp. 6365-6378, 2016. [Crossref]
[31] A. Nokhodchi, C.M. Hentzschel, C.S. Leopold, "Drug release from liquisolid systems: Speed it up, slow it down" Expert Opin. Drug Deliv., vol. 8, pp. 191-205, 2011. [Crossref] [PubMed]
[32] D. Chopra, D. Madhab, P. Sahu, "Improvement of oral bioavailability of azilsartan medoxomil by lipid based liquisolid compacts: In vitro and in vivo evaluation" Int. Res. J. Pharm., vol. 9, pp. 134-139, 2019. [Crossref]
[33] K.P.R. Chowdary, S. Srinivasan, "Effects of Cyclodextrins, Tween-80 and PVP on the Solubility and Dissolution Rate of Etoricoxib" J. Pharm. Sci. Res, vol. 3, pp. 1344-1348, 2011. Available online: https://www.jpsr.pharmainfo.in/Documents/Volumes/Vol3Issue07/jpsr%2003110709.pdf.
[34] S. Kumar, S. Kasimedu, "Dissolution Enhancement of Poorly Soluble Drugs by Using Complexation Technique -A Review" Pharm. Sci. Res., vol. 5, pp. 120-124, 2013. Available online: https://www.jpsr.pharmainfo.in/Documents/Volumes/vol5issue05/jpsr05051305.pdf.
[35] S. Noreen, I. Maqbool, M. Ijaz, S. Tanveer, "Cyclodextrin Inclusion Complexes: Novel Techniques to Improve Solubility of Poorly Soluble Drugs: A Review" Glob. Pharm. Sci. Rev., vol. I, pp. 29-34, 2016. [Crossref]
[36] O. Rajabi, R. Salari, S. Tayyari, "Study of structure and properties of Lidocaine: Hydroxypropyl-ß-cyclodxtrin inclusion complex" J. Pharm. Res., vol. 4, pp. 1562-1563, 2011. Available online: https://profdoc.um.ac.ir/paper-abstract-1028612.html.
[37] S. Shivanand, "Influence of method of preparation on solubility, physicochemical properties and invitro release profile of simvastatin cyclodextrin inclusion complexes: A comparative study" Int. J. Curr. Pharm. Res., vol. 2, pp. 7-12, 2010. Available online: https://sphinxsai.com/sphinxsaivol_2no.1/chemtech_vol_2no.1/ChemTech_Vol_2No.1PDF/CT=89%20(562-571).pdf.
[38] Y. He, W. Zhang, T. Guo, G. Zhang, W. Qin, L. Zhang, et al., "Drug nanoclusters formed in confined nano-cages of CD-MOF: Dramatic enhancement of solubility and bioavailability of azilsartan" Acta Pharm. Sin. B, vol. 9, pp. 97-106, 2018. [Crossref] [PubMed]
[39] R.K. Maheshwari, V.K. Srivastav, R.P. Prajapat, A. Jain, P. Kamaria, S. Sahu, "New spectrophotometric estimation of ornidazole tablets employing urea as a hydrotropic solubilizing additive" Indian J. Pharm. Sci., vol. 72, pp. 258-261, 2010. [Crossref] [PubMed]
[40] R. Maheshwari, M. Rajput, S. Sinha, "Ecofriendly spectrophotometric estimation of tinidazole in tablets using lignocaine HCL as a hydrotropic solubilising agent" Asian J. Pharm., vol. 3, pp. 319-321, 2009. [Crossref]
[41] J.N. Joshi, S. Nainwal, A. Vikas, "A review on hydrotropy: A potential approach for the solubility enhancement of poorly soluble drug" Asian J. Pharm. Clin. Res., pp. 19-26, 2019. [Crossref]
[42] A. Mahapatra, V. Patil, R. Patil, "Solubility Enhancement of Poorly soluble Drugs by using Novel Techniques: A Comprehensive Review" Int. J. PharmTech Res., vol. 13, pp. 80-93, 2020. [Crossref]
[43] V. Bhamare, P. Deore, S. Katti, "Application of mixed hydrotropy for the solubility enhancement" World J. Pharm. Pharm. Sci., vol. 10, pp. 1163-1176, 2021.
[44] K.S. Surwade, R.B. Saudagar, "Solubility enhancement of azilsartan medoxomil using mixed hydrotropy" World J. Pharm. Pharm. Sci., vol. 4, pp. 1167-1179, 2015. Available online: https://www.wjpps.com/wjpps_controller/abstract_id/3423.
[45] M.B. McGuckin, J.W. Wang, R. Ghanma, N.Y. Qin, S.D. Palma, R.F. Donnelly, et al., "Nanocrystals as a master key to deliver hydrophobic drugs via multiple administration routes" J. Control Release, vol. 345, pp. 334-353, 2022. [Crossref] [PubMed]
[46] Y. He, Z. Ye, X. Liu, Z. Wei, F. Qiu, H.-F. Li, et al., "Can machine learning predict drug nanocrystals?" J. Control Release, vol. 322, pp. 274-285, 2020. [Crossref]
[47] B. Xie, Y. Liu, X. Li, P. Yang, W. He, "Solubilization techniques used for poorly water-soluble drugs" Acta Pharm. Sin. B, vol. 14, pp. 4683-4716, 2024. [Crossref] [PubMed]
[48] Z.H. Tian, Y.P. Mai, T.T. Meng, S.J. Ma, G.J. Gou, J.H. Yang, "Nanocrystals for improving oral bioavailability of drugs: Intestinal transport mechanisms and influencing factors" AAPS PharmSciTech, vol. 22, p. 179, 2021. [Crossref]
[49] T.R. Meola, T.J. Dening, C.A. Prestidge, "Nanocrystal-silica-lipid hybrid particles for the improved oral delivery of ziprasidone in vitro" Eur. J. Pharm. Biopharm., vol. 129, pp. 145-153, 2018. [Crossref]
[50] J. Ma, Y. Yang, Y. Sun, J. Sun, "Optimization, characterization and in vitro/vivo evaluation of azilsartan nanocrystals" Asian J. Pharm. Sci., vol. 12, pp. 344-352, 2017. [Crossref] [PubMed]
[51] L. Gao, X. Zhang, "Synthesis of Two Novel Azilsartan Cocrystals: Preparation, Physicochemical Characterization and Solubility Studies" Crystals, vol. 10, 2020. [Crossref]
We use cookies to improve your experience on our site. By continuing to use our site, you accept our use of cookies. Learn more