
APA Style
Aanchal Siwach, Vishakha Baliyan, Ayushi Sharma, Shraddha Ahlawat, Shikha Chaudhary, Eliza Chakraborty. (2025). Current Standing and Future Potential of 3D Bioprinting and Biomaterials. Biomaterials Connect, 2 (Article ID: 0015). https://doi.org/10.69709/BIOMATC.2025.198090MLA Style
Aanchal Siwach, Vishakha Baliyan, Ayushi Sharma, Shraddha Ahlawat, Shikha Chaudhary, Eliza Chakraborty. "Current Standing and Future Potential of 3D Bioprinting and Biomaterials". Biomaterials Connect, vol. 2, 2025, Article ID: 0015, https://doi.org/10.69709/BIOMATC.2025.198090.Chicago Style
Aanchal Siwach, Vishakha Baliyan, Ayushi Sharma, Shraddha Ahlawat, Shikha Chaudhary, Eliza Chakraborty. 2025. "Current Standing and Future Potential of 3D Bioprinting and Biomaterials." Biomaterials Connect 2 (2025): 0015. https://doi.org/10.69709/BIOMATC.2025.198090.
 ACCESS
Review Article
ACCESS
Review Article
Volume 2, Article ID: 2025.0015

Aanchal Siwach
Aanchalsiwach01@gmail.com

Vishakha Baliyan
v.b.vishakha03@gmail.com

Ayushi Sharma
sharmaayushi013@gmail.com

Shraddha Ahlawat
Shraddhaahlwat@gmail.com

Shikha Chaudhary

Eliza Chakraborty
eliza.chakraborty@miet.ac.in
1 Medical Translational Biotechnology Lab, Meerut Institute of Engineering & Technology, Meerut 250005, Uttar Pradesh, India
2 DST-FIST Center Sponsored by Ministry of Science & Technology, Government of India, Miet, Meerut 250002, Uttar Pradesh, India
3 ImmunoScience India Private Limited, Pune 411 004, Maharashtra, India
* Author to whom correspondence should be addressed
Received: 23 Dec 2024 Accepted: 11 Apr 2025 Published: 15 May 2025
Biomaterials play a pivotal role in advancing tissue engineering, offering significant potential in 3D bioprinting and translational medicine. Their development has led to the creation of innovative solutions in medical devices, dental implants, and prosthetics, where they serve to restore function and enhance patient quality of life. The success of these biomaterials is primarily dependent on important characteristics that guarantee their safe integration with the body and promote tissue regeneration, such as biocompatibility, biodegradability, and bifunctionality. This review explores recent advancements in these areas, with a particular focus on the role of nanotechnology in enhancing biomaterial properties. Biomaterials have opened many avenues by improving mechanical, biological, and functional properties for targeted treatments, precision therapies, improved therapeutic effectiveness, and better results. Additionally, the rise of 3D printing technologies has transformed the design and fabrication of biomaterials, enabling the production of customized implants and scaffolds with intricate geometries suited to individual patient needs. This review examines the current state of research, challenges faced in the field, and emerging trends in the integration of nanotechnology and additive manufacturing to push the boundaries of biomaterial applications in tissue engineering and regenerative medicine.
A biomaterial is any material or substance that is designed to interact with biological systems therapeutically and medicinally. Implants, prostheses, medical devices, drug delivery systems, and tissue engineering are among the common applications for these materials. Biomaterials, which can be produced in a lab or obtained from natural sources, are designed to interact with living tissue to replace or restore a biological function that has been disrupted. These biomaterials, regardless of their origin, are designed to help certain cell populations adhere, maintain, proliferate, and occasionally differentiate [1]. In contrast to cells cultivated in two-dimensional (2D) monolayers, recent research has demonstrated that 3D spheroids exhibit improved biological functions. This is probably because 3D spheroid culture replicates a native-like tissue microenvironment that enables direct cell-cell signaling and cell-matrix interactions [2]. Three-dimensional (3D) bioprinting offers the potential to produce custom tissue-engineered structures, eliminating the complications associated with donor sites, enabling the production of intricate organs tailored to individual needs, and ushering in a breakthrough in biological sciences and medical research [3]. It is an emerging innovative technology revolutionizing the field of biomedical applications by combining engineering, manufacturing, art, education, and medicine. This process involves incorporating the cells with biocompatible materials to design the required tissue or organ model in situ for various in vivo applications. To generate the desired 3D structure, 3D printing involves the layer-by-layer deposition of bioinks (such as tissue spheroids, microcarriers, cell pellets, etc.) in a specially planned manner, guided by a software-supported system. Previously, this method was limited to using molds to create the required three-dimensional structures out of biological materials [4]. The rapidly developing technique of 3D printing in the medical field enables the creation of comparable structures from various cellular, tissue, and organ models. It is employed in the production of prosthetic devices, orthoses, and scaffolds for various biomedical uses. A variety of natural and synthetic materials are used to print 3D structures. In addition to being mechanically reinforced, possessing tunable mechanical properties, being biocompatible, having adequate degradation kinetics, having nontoxic degradation byproducts, biomimetics, and the ability to release therapeutic agents in a controlled manner, printable materials should also have sufficient viscosity and the ability to form 3D structures in a specific amount of time. Biomaterials must also be easily produced and processed, cost-effective, and commercially available. Natural polymers such as alginate, gelatin, chitosan, collagen, silk, hyaluronic acid (HA), fibrinogen, and agar are used to create 3D models, either alone or combined with other polymers or fillers [4]. 3D bioprinting, a novel technique with huge potential for personalized medicine, has been explored to investigate the relationship between biomaterials and regenerative medicine. This cutting-edge technique is being explored to develop patient-specific tissue structures for improved therapeutic applications. The quick advancement in this discipline highlights its potential to transform tissue engineering and drug discovery. However, challenges remain in creating anatomically realistic structures with fully developed biological functions. Another area of biomaterials research is microfluidic organ-on-a-chip devices, which serve as platforms for incredibly accurate modeling of intricate organ systems. Recent applications of microfluidic organ-on-a-chip technology have included the use of sophisticated ex vivo models to enhance our understanding of tissue engineering. Researchers are well-positioned to ascertain the complexities of human physiology and create innovative approaches for disease modeling and drug screening [5,6,7]. Regenerative medicine has recently emerged as one of the most promising fields in biomedical engineering. Advancing regenerative medicine requires enhancing cell activity, as the natural ability of tissues to heal is often insufficient for effective cell migration, proliferation, and differentiation in damaged or injured tissues. If scientific technology can improve the natural healing potential of damaged cells, “patient-friendly” tissue regeneration may become a reality, as shown in Figure 1. For in vitro research, the use of living cells with viable and functional properties is recommended. Typically, cells are cultivated in polystyrene-based dishes—an artificial environment that significantly differs from the native tissue conditions. In contrast to the in vivo setting, where cells interact well with one another and with the extracellular matrix (ECM) to promote differentiation, proliferation, metabolism, and cytokine secretion, the altered cell state in vitro cultures often results in decreased cell activity. Due to variations in cell state or activity, the drug effect shown under in vitro drug screening settings may not necessarily be the same as that observed in a preclinical or clinical trial. Effective drug evaluation requires the use of high-activity cells, as their responses are more likely to reflect actual physiological outcomes. Therefore, improving cell function and activity in both in vivo and in vitro settings is crucial for the advancement of regenerative medicine [8]. For in vitro cell culture, biologists and bioengineers have explored a variety of three-dimensional (3D) scaffolds that mimic elements of the natural cellular microenvironment. Of these, hydrogels—crosslinked networks with high water content—have proven particularly effective as 3D cell culture matrices. Nowadays, a wide range of hydrogels is used for mammalian cell culture, ranging from entirely natural to entirely synthetic materials. Each hydrogel system has its advantages and limitations. As the field advances, there is a growing demand for matrices that combine the benefits of both synthetic and natural hydrogels. Additionally, identifying the crucial biophysical and biochemical signals necessary for inclusion in these artificial extracellular matrix (ECM) analogs remains an important area of research [11]. Although 3D bioprinting is capable of producing tissue and organ structures with ease, improvements are still needed in areas such as bioink development and the commercialization of 3D printed products. For urgent medical demands, this technique can help create more intricate 3D structures tailored to each patient. It offers many benefits, including control over mechanical properties, biodegradation, usage of particular cell lines, printing modes, and design flexibility. Cell-laden hydrogels are one of the most popular methods for creating these three-dimensional structures. This review has covered the many available bioinks, their characteristics, and the numerous selection criteria for bioinks. The process of creating the ideal bioink is still ongoing, but because of the tremendous contributions made by researchers all over the world, it may soon be feasible to apply this technology in commercial applications. ECM-based bioinks, decellularized bioinks, cell aggregates, and spheroids are also demonstrating encouraging outcomes in the production of functional tissues and organs using 3D bioprinting technology, even though cell-laden biomaterial bioinks remain widely used. However, these methods require a large number of specific cells, which restricts their application in many organs and tissues. In addition to bioinks, it is believed that the development of affordable, high-resolution will further advance the field. The selection and use of bioinks have become more promising with the recent reports of numerous novel biomaterials with supramolecular functionality, reversible crosslinking polymers, and stimuli-responsive hydrogels. Bioinks and 3D bioprinting have a bright future ahead, leading to the creation of sophisticated patient-specific tissues, organs, and devices [12].![Figure 1: <p>This figure illustrates how biomaterials enhance cell activity in regenerative medicine. In in vitro research, they improve traditional culture conditions by mimicking in vivo environments. In in vivo therapy, biomaterials aid cell seeding in damaged tissues, promoting regeneration [<a href="#ref9">9</a>,<a href="#ref10">10</a>]. Courtesy of Murthy et al., ijrpr, October 2023. Created using MS Paint.</p>](/uploads/source/articles/biomaterials-connect/2025/volume2/20250015/image001.png)
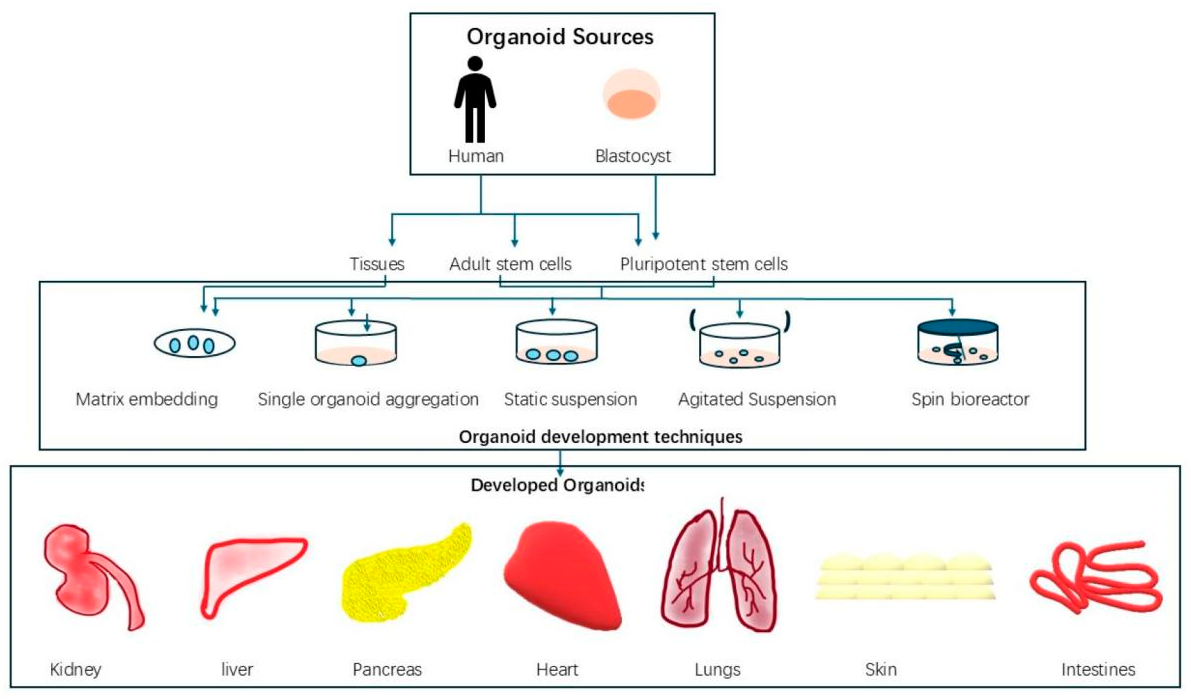
2.1. Biomaterials for 3D Printing Three-dimensional (3D) printing can create complicated structures and patient-specific designs, while also enabling fast and cost-effective production. It is frequently employed in tissue engineering to create scaffolds and devices. However, the limited variety of “biomaterial inks” remains a major obstacle in biomanufacturing [13]. Biomaterials that interact with biological systems are categorized according to their composition, biodegradability, and modification generations [14]. Concerning Food and Drug Administration (FDA) approval, every biomaterial has distinct characteristics, processing techniques, and cell interactions [15]. The technology employed determines printability; inks made of hydrogels, polymers, and ceramics are among the options [13]. While bio-paper (hydrogel) was created to incorporate living cells or spheroids during bioprinting, bio-ink, which was first associated with organ printing, comprises bioactive chemicals, biomaterials, and cells to create scaffolds [16]. Hydrogels are ideal for scaffolds in tissue engineering because they are primarily water-based, biocompatible, adjustable, and resemble the extracellular matrix (ECM) [17]. Hydrogels can be categorized as natural, synthetic, or hybrid materials [18]. 2.2. Types of Biomaterials Biomaterials can be classified into different categories as described in Figure 2. Natural biomaterials are derived from plants, animals (xenogenic), or humans (allogenic) and can exist as non-viable decellularized extracellular matrix (ECM) or derivatives, or as viable living cells (allogenic or autologous). These biomaterials mimic native tissue structures, supporting tissue reconstruction, repair, and regeneration [19]. Synthetic biomaterials are classified into four categories: (1) polymers, including natural types like collagen, sodium alginate, and cellulose, and synthetic types such as silicone rubber, PMMA, PVC, and co-PLGA; (2) metals; (3) ceramics; and (4) composites. Metals, such as titanium alloys, stainless steel, and Co–Cr alloys, are widely used in dental and orthopedic fields. Ceramics, like calcium phosphates (CaP), alumina (Al2O3), and bioglass, are used for hard tissue repair, especially as coatings or in non-load-bearing applications. Polymer–ceramic composites are the most widely utilized type among composite biomaterials [20]. 2.3. Properties of Biomaterials Biomaterials exhibit unique physical, chemical, and biological properties influenced by their surface and bulk characteristics, as surface properties differ due to unsatisfied bonds at the outer layer [20]. Physically, they require mechanical strength (e.g., titanium alloys for implants), suitable surface morphology (e.g., porous hydrogels for cell growth), and controlled degradation rates (e.g., PLGA for scaffolding) [17,18,20]. Chemically, biomaterials offer tunable compositions (e.g., collagen, PLA), stability under physiological conditions (e.g., stainless steel, alumina), and functionalized surfaces for tissue bonding (e.g., bioactive glass) [10,16,17]. Biologically, they must ensure biocompatibility (e.g., hydrogels mimicking ECM), bioactivity for regeneration (e.g., calcium phosphates for osteogenesis), and low immunogenicity (e.g., processed collagen) [14,20,22]. The characteristics of biomaterials are defined by their biocompatibility, mechanical strength, surface properties, degradability, chemical stability, bioactivity, and low immunogenicity, ensuring their effectiveness in biomedical applications. Hydrogels mimic the ECM to support cell attachment [22], while titanium alloys provide strength for orthopedic implants [20]. Surface porosity enhances tissue integration, as seen in hydrogels [17], and PLGA scaffolds offer controlled degradation for tissue replacement [18]. Corrosion-resistant materials like stainless steel and alumina ensure stability [20], while bioactive calcium phosphates promote bone regeneration [20]. Processed collagen minimizes immune reactions, enhancing biocompatibility [14]. These properties make biomaterials essential for tissue engineering and regenerative medicine. 2.4. Advantages of Biomaterials Biomaterials have several major benefits for tissue culture, including aiding cell adhesion and proliferation by imitating the extracellular matrix (ECM), with hydrogels providing an ideal environment for tissue formation [22]. For long-term culture and implantation, these materials are essential because they are biocompatible, non-toxic, and well-tolerated by live tissues, reducing immunological reactions [14]. The use of biomaterials to facilitate the creation of robust and useful 3D structures significantly increases the efficiency and adaptability of 3D bioprinting. Nanomaterials promote vascularization, ensuring that 3D-printed tissues receive adequate nutrients and oxygen, which are essential for their viability and function. They are also used to promote healing and regeneration in 3D-printed tissues, reducing the need for animal models and facilitating more ethical and effective clinical and research applications. By altering mechanical strength and rates of degradation, biomaterials can be tailored to match the unique requirements of various tissues, including bone and cartilage [19]. Bioactive compounds, such as calcium phosphates, promote tissue regeneration by releasing osteogenic ions, while materials like PLGA degrade in a controlled manner, enabling natural tissue to gradually replace the scaffold [18,20]. 2.5. 3D Bioprinting Techniques 3D Bioprinting is a technology where a 3D bioprinter uses living cells, deposited layer by layer, to create biological tissue structures. Bioinks, which are soft biomaterials loaded with living cells used to create biological structures, are typically used by bioprinters to accomplish this goal. Another way to support and shield cells vertically during printing is to utilize secondary dissolvable materials. Normally, 3D bioprinting relies on precisely placing biological components, biochemicals, and living cells layer by layer through spatial management of the functional components of the 3D construction. 3D bioprinting relies on three basic techniques: (a) biomimicry or biomimetics, (b) autonomous self-assembly, and(c) mini-tissue building blocks [23]. Thus, a key component of 3D bioprinting is the techniques used to design and build the architecture; different bioprinting methodologies depend on fundamental working principles, including different techniques [24]. Various features and applications of different types of bioprinting techniques are described precisely in Table 1. Features and applications of different strategies of 3D bioprinting [24]. 2.5.1. Extrusion-Based Bioprinting Extrusion-based bioprinters typically use a mechanical screw or compressed air to extrude the material, depending on its viscosity. Extrusion-based printers can print more viscous materials and have higher cell densities than inkjet and laser-based printers. Lower-viscosity materials are preferable for biological activities, whereas greater-viscosity materials give scaffolds better structural support. This is because, while extrusion printers can print a wide variety of materials, the cells undergo additional mechanical pressures during the printing process that are proportionate to the printed part’s pressure, viscosity, form, and speed [44]. This occurs because cells experience varying velocity gradients based on their radial position, due to pressurized bioinks flowing through a nozzle with fixed geometry. Material slippage along a plane parallel to the direction of stress results in the cells experiencing the largest shear stress and velocity gradient near the nozzle walls. In extrusion-based printing, this is the main factor causing cell deformation and apoptosis [45]. 2.5.2. Laser-Assisted Bioprinting The purpose of laser-assisted printing was to deposit metals onto an optically transparent substrate. Then, in 2000, Odde and Renn, along with Ren, used this method to print the spinal cords of embryonic chicks, demonstrating that it can be used to precisely create arrays of hundreds of cells at the micrometer scale. In contemporary laser-assisted bioprinting procedures, cells are deposited from a ribbon or donor slide onto the receiving substrate (as shown in Figure 3A) below in a predetermined pattern using laser pulses [3]. A layer of glass, metal, and bio-ink typically makes up the donor slide. When the laser strikes the metal, the metal absorbs the energy, causing the hydrogel to evaporate and releasing the freshly separated cells. Laser-assisted bioprinting typically offers the highest yield (cell viability >95%) and resolutions of up to one cell among the bioprinting methods covered. This is because various printing parameters, such as laser energy, surface tension, wettability, the air gap between the donor slide and substrate, and bioink viscosity, can be precisely adjusted [46]. 2.5.3. Inkjet Bioprinting The Hewlett-Packard Company developed inkjet technology, a 2D printing technique, in the 1970s, and it was the first bioprinting technology of additive manufacturing. In 1992, a chamber and a z-axis-moving elevator platform were added to this device, creating a 3D bioprinting system [43,47]. For tissue engineering applications, thermal and piezoelectric inkjet bioprinters are more commonly utilized. In thermal inkjet bioprinting, an ink cartridge is filled with a prepolymer solution called bioink, which may contain cells. The cartridge is inserted into the computer-controlled printer head, where tiny ink droplets are released with the help of small air bubbles generated by the heat from the printer head, as shown in Figure 3B. The gradient of applied temperature, the frequency of the current pulse, and the viscosity of the ink can all alter the size of the droplet [48]. 2.5.4. Coaxial Bioprinting Coaxial bioprinting, which uses a coaxial nozzle (as seen in Figure 3C), is a relatively recent bioprinting technique [49]. This technique is particularly well-suited for creating tissue with integrated microchannels or vertical structures with heterogeneous properties, as the sheath and core channels can be supplied with biomaterials that possess distinct physicochemical characteristics [24]. Various tissues can be created with coaxial bioprinting when mechanical parameters such as toughness, shear force, viscosity, and compression are adjusted by selecting the appropriate biomaterials. Materials suitable for bioprinting vascularized structures include alginate and gelatin methacryloyl (GelMA)/gelatin [50,51,52]. 2.5.5. Acoustic Bioprinting The new technique, known as “acoustic bioprinting,” is founded on the idea of “acoustic droplet ejection.” The focus of the ultrasonic signal is near the air-liquid contact, and the acoustic printer uses an open cartridge. A flow against the surface and the creation of a dome on the liquid surface result from the acoustic streaming effect. When the sound pressure from the ultrasonic signal and the kinetic flow energy are generated and overcome the surface tension of the liquid, droplets of liquid are ejected. The substrate collects the liquid droplets [53]. The printer can print single cells, high-density cells, and even cell spheroids with flexibility because it doesn’t have a nozzle, which also reduces cell damage. When it comes to high resolution, acoustic bioprinting offers the benefit of easy and adaptable placement of tumor cells, normal cells, fibroblasts, and extracellular matrix, which greatly contributes to the replication of the tumor microenvironment. 2.5.6. Magnetic Bioprinting Haisler et al. (2013) reported a magnetic levitation-based 3D culture technique in which cells were made magnetic by attaching magnetic nanoparticles to them [54]. An external magnetic field may lift and concentrate the magnetic cells at the air-liquid interface once they are resuspended in the medium. The cells were gathered throughout this procedure to create three-dimensional cultures. This technique served as the basis for the development of magnetic 3D bioprinting. Without the use of additional synthetic protein substrates, this method can produce an endogenous extracellular matrix and offer precise spatial control. It can also print a high-throughput pattern of many tissue-like structures. Tissue engineering has used similar technologies for a variety of purposes, including aortic valve, lungs, adipose tissue [55], prostate tumor, and so on. The capacity of magnetic bioprinting to create native-like tissue in a high-throughput manner is its primary benefit. In the first extensive screening project, Fernandez-Vega et al. used this technique to finish high-throughput screening (HTS). 2.5.7. Digital Light Projection Bioprinting Figure 4 illustrates digital light projection (DLP) bioprinting, which is comparable to laser-assisted printing, except it uses a UV source to beam light onto a liquid resin, curing it into a series of square 3D pixels. In contrast to the majority of similar laser-assisted bioprinters, newer DLP bioprinters can produce models with much higher fidelity (<20 microns) at quicker fabrication speeds because the print resolution is determined by a single pixel [56]. Additionally, DLP printing is more adaptable and permits variations in the UV light intensity for different resins, in contrast to laser-assisted printing, which employs a fixed-intensity laser beam [57]. This adaptability has contributed to the development of novel techniques for creating different microtissue models in the field, including tumor organoid and tumor-on-chip modeling. Although DLP systems can optically create simple microvascular models, significant advancements in light-absorbing bioinks are required before multi-material DLP bioprinters and intricate vasculature become widespread [58]. 2.5.8. Droplet-Based Bioprinting In contrast to extrusion-based bioprinting, droplet-based bioprinting uses microscopic ink droplets produced mainly by thermal, piezoelectric, or electromagnetic actuators to replicate designs onto a substrate without making contact. These actuators, with resolutions ranging from 20 to 100 microns, allow for selective control of droplet flow. Although the production time of droplet-based bioprinting is substantially slower than extrusion-based bioprinting, it offers higher accuracy and precision [60]. 2.6. FRESH (Freeform Reversible Embedding of Suspended Hydrogels) The freeform reversible embedding of suspended hydrogels (FRESH) is a newly developed 3D bioprinting method. FRESH is a very flexible and affordable biological AM platform that uses a thermoreversible support bath to allow hydrogel deposition in intricate, three-dimensional biological structures. It is implemented with open-source tools. The fundamental novelty of FRESH is the deposition and embedding of the hydrogel(s) being printed within a second hydrogel support bath, which preserves the intended structure throughout the print process and majorly enhances print fidelity [61,62]. 2.7. Contemporary Overview The field of 3D bioprinting, a new area of additive manufacturing (AM) technology, has significant room for growth. The scientific literature has shown a remarkable amount of interest in this field in recent years, drawing numerous inventors and generating new, intriguing markets. All of these indicators suggest that we may be witnessing the development of a long-term research avenue. The purpose of this study was to give the reader a thorough overview of the academic and industry landscape of 3D bioprinting, rather than to prepare another review paper. In this way, experienced academics can get an updated snapshot of the current state of this rapidly evolving field, and new researchers can use it as a compass to explore exciting emerging technologies [9]. As 3D bioprinting evolves, nanomaterials have become increasingly important in regenerative medicine [10,63]. They are being used to create scaffolds that mimic the extracellular matrix found in nature, which is essential for improving tissue growth and healing. These advanced materials are helping to enhance the quality and effectiveness of bio-printed tissues. The volumetric printing technique, also known as holographic printing or multi-beam additive manufacturing, offers isotropic mechanical properties and high printing speeds [64,65]. By irradiating a volume of photosensitive resin from various angles, volumetric printing creates the complete 3D object at once, unlike the traditional layer-by-layer method. This technology can be applied in two ways: (1) tomographic reconstruction, which projects a sequence of two-dimensional light patterns that are calculated using a Radon transform synchronously into a rotating resin container [65], and (2) using a system architecture that consists of mirrors that split a single light beam into three orthogonal beams, from which a holographic figure is projected into a photosensitive resin to create the desired object [64]. Printing time can be significantly reduced by fabricating the desired 3D structure in a single step by superposing many 2D images of the same object taken from various angles. By delivering 2D light patterns to each plane of the photosensitive resin, the patterns accumulate to create the necessary 3D structure in a single step, significantly cutting down on printing time. Each light exposure does not provide enough light doses to crosslink the photosensitive resin. After being exposed to the projected 2D light patterns repeatedly, light doses accumulate in particular regions that are specified by the intended 3D geometry. Localized regions where the total absorbed light dose beyond the crosslinking threshold subsequently experience photopolymerization [66]. 2.8. Disease Modeling Using 3D in Vitro Models Before the invention of techniques for 3D in vitro model development, animal models like pigs, rabbits, rodents, and monkeys were mainly preferred for research and therapeutic studies due to their genetic similarity with humans, and many other factors like size, structure, gestation period, and high population. Conventional 2D cell culture models have limitations because they cannot replicate the in vivo extracellular matrix (ECM) interactions and microenvironment. As a result, research in drug discovery, cancer studies, and human disease modeling requires more advanced cellular systems. To address this, scientists have developed 3D cell culture models. Spheroids are developed from a single cell type with low structural complexity and hence preferred for drug screening studies whereas organoids can be developed from adult, and pluripotent stem cells and hence mimic the histological and genetic features of the source sample, and properties like ease of generation, long term culture ability and cryopreservation makes them suitable for various research applications [10,67,68]. Today, the personalized 3D in vitro models (as shown in Figure 5)—organoids and spheroids have completely revolutionized the area of research and therapeutics. Between 2024 and 2034, globally, the organoids and spheroids products market are predicted to grow with a 23.12% compound annual growth rate from USD 0.781 billion in 2023 to USD 7.70 billion by 2034 [69]. A few of the organoid disease models are discussed below. 2.8.1. Brain Disease Modeling In early 2000, embryoid bodies generated from embryonic stem cells were used to form in vitro brain models [70]. By 2013, a cerebral organoid generation system was developed, and since then, new procedures and techniques have been introduced to generate brain organoids that closely mimic the human brain. Using brain organoids developed from different types of stem cells (induced pluripotent, pluripotent, embryonic) as disease models breakthrough achievements have been reported for brain disorders like microcephaly, macrocephaly, autism spectrum disorder, Schizophrenia, Rett syndrome, Sandhoff disease, Miller-Dieker syndrome, Zika virus infection, Alzheimer’s, and Parkinson’s disease [67]. 2.8.2. Cancer Modeling Tumor organoids, also known as tumoroids, are being used to understand, investigate, and cure cancer. The genetic mutations in the DNA result in unusual cellular processes. Using gene editing techniques to make genetic alterations in healthy organoids and tumoroids helps identify: (a) the signaling cascade affecting oncogenic phenotypes, and (b) events associated with tumor initiation, development, and progression [68]. So far, human tumoroids have been developed from esophageal, stomach, lung, colon, liver, pancreas, brain, prostate, breast, bladder, and endometrial tumor samples. Recently, in a cancer drug trial, a 90% similar drug response was recorded in patients and organoid cultures [67]. 2.8.3. Cardiac Disease Modeling A major limitation in cardiac disease drug discovery and the study of congenital heart defects is the limited supply of cardiac tissue samples and inadequate in vitro culture conditions. In the past decade, researchers have made significant efforts to develop cardiac organoid models using tissue engineering techniques. However, these models have often been expensive, labor-intensive, difficult to scale, and inconsistent with the heart’s native cell composition and structure. Recently, self-assembling organoid techniques have been developed to generate self-assembling cardiac organoids using stem cells. Most notably, Lewis-Israeli et al. developed a self-assembling cardiac organoid using human pluripotent stem cells and a three-step Wnt signaling cascade modulation strategy [71]. Human cardiac organoids have been developed and used as disease models to investigate Barth syndrome, Dilated cardiomyopathy, Hypertrophic cardiomyopathy, Acute myocardial infarction, and Duchenne muscular dystrophy [67]. 2.9. Challenges and Their Solution Dynamic processes like tissue formation, disease progression, and recovery require adaptable in vitro models with tunable biochemical, structural, and mechanical properties. Traditional hydrogels lack this flexibility, but composite hydrogels with reversible crosslinking offer enhanced control over degradation and mechanical properties, improving cell activity and supporting long-term culture [72,73]. Challenges in 3D bioprinting vascular networks include cell-material compatibility and maturation time [74,75]. Bioreactors sustain tissue activity, while self-sacrificing materials may facilitate large vessel printing, with capillaries forming through stem cell differentiation and smaller vessels forming via host expansion. Vascularization strategies involve integrating angiogenic factors, pre-vascularization techniques, and co-culture systems, requiring careful optimization of cell types, seeding methods, and microenvironments. For instance, VEGF-infused electrospun PCL scaffolds have successfully promoted endothelial cell growth [27,76,77]. Current 3D in vitro models struggle to replicate the intricate microenvironments of tissues and diseases, including their complex structures and diverse cell types. A systematic approach using big data and AI to analyze hydrogel databases can identify key factors for developing more accurate models, enhancing tissue niches, and guiding cell behavior [4]. For example, Lee et al. created a 3D-printed liver-on-a-chip using porcine liver dECM as bioink, incorporating hepatocytes, endothelial cells, and cholangiocytes to simulate liver functions. The inclusion of the biliary system improved liver function and drug sensitivity. They also developed a liver fibrosis model using dECM, gelatin bioink, and activated stellate cells to mimic fibrosis characteristics like collagen deposition and reduced liver functionality [78,79,80]. Balancing bioink’s tissue regeneration ability with printability is difficult. Increasing concentration or crosslinking density enhances printability but can reduce biocompatibility, necessitating optimization for customized performance. Sophisticated bioprinting methods are essential to extend the bio-fabrication window. Advances in bioinks, like stimuli-responsive hydrogels with low initial viscosity, enhance shape fidelity and printability, enabling precise tissue constructs [81]. Decellularized bioinks face challenges due to low mechanical properties and poor deposition ability in 3D bioprinting [82,83]. Strategies to address these include novel printing techniques and adding crosslinking agents to dECM bioinks. Overcoming precise bioink deposition and complex structure creation, such as overhangs, involves using thermoresponsive hydrogels like gelatin and PF-127 to improve deposition capacity. These hydrogels also support sacrificial bioinks for intricate, stable 3D architectures [81]. Photoresponsive hydrogels address crosslinking management and enhance the printability of low-viscosity bioinks by using light to precisely control crosslinking, thereby increasing bioink stability and resolution. Shear-responsive bioinks, like silk fibroin, avoid harmful crosslinking agents, enhancing cell compatibility and allowing control over mechanical properties and viscosity [81,84,85]. Single-component hydrogel bioinks often fail to balance printability and cellular function, whereas multi-component bioinks combine benefits to improve deposition, tissue specificity, and viscosity [81,86]. Isotropic hydrogels do not replicate the anisotropic nature of native ECM. Future research should focus on hydrogels with gradient, stimuli-responsive, and ECM-inspired properties for dynamic control [72]. Traditional hydrogels are not adaptable, but composite hydrogels with reversible crosslinking offer controlled degradation and mechanical tuning, supporting cell activity and long-term culture [72]. Static cellular models cannot mimic dynamic physiological conditions such as blood flow and interstitial fluid movement, limiting their accuracy. Incorporating fluidic systems enhances physiological relevance. Microfluidics-based organ-on-a-chip (OOC) systems simulate dynamic tissue environments more realistically, improving model fidelity [27,72,79,87]. Integrating multiple organs and their interactions in one model is challenging due to complex molecular signaling affecting organ functions. For instance, a drug targeting the liver can impact the heart and lungs. In cancer research, tracking malignant cell spread requires interconnected tissue models with circulatory systems. Despite progress in multi-organ platforms, developing 3D systems that accurately replicate these interactions is crucial for reliable disease modeling and drug testing [88,89]. Scientists have successfully produced organoids such as the brain, lungs, heart, kidneys, and others using an advanced multi-material printer capable of printing with seven types of bioinks. The development of in situ bioprinting technology addresses the challenges associated with manual implantation of prefabricated tissue constructs [90,91,92]. For instance, Albanna et al. developed portable skin bioprinting technology for on-site wound care, demonstrating faster re-epithelialization, reduced contraction, and improved wound healing [93]. With ongoing advancements in biomaterials, bioprinting resolution, and integrated monitoring systems, in situ bioprinting shows great potential for constructing functional tissues directly within the body [74]. Creating 3D models that mimic the intricate structure of living tissues is a complex task, but 3D bioprinting provides a viable approach. This technique enables precise control over the placement of tissue components at the nanoscale and microscale, resulting in biomimetic models. The process involves printing 2D slices derived from a 3D CAD model [94,95]. While hydrogel inks and biomaterials used in this process are biocompatible, they often lack crucial bioactivity and mechanical characteristics, such as sites for cell adhesion and degradation. To overcome this limitation, bioactive sequences like RGD and MMP-sensitive sequences are integrated to enhance bioactivity [96,97]. Natural protein hydrogels, including gelatin, fibrin, and collagen, inherently possess cell-binding and degradable sites but are hampered by weak mechanical properties. To boost bioactivity, particularly in osteo- and osteochondral applications, bioactive particles such as calcium phosphates (tricalcium phosphate, hydroxyapatite) are employed [97,98,99]. Furthermore, various crosslinking methods are utilized to manage mechanical stress and fine-tune bioprinting parameters [97,100,101].![Figure 2: <p>Types of biomaterials [<a href="#ref20">20</a>,<a href="#ref21">21</a>].</p>](/uploads/source/articles/biomaterials-connect/2025/volume2/20250015/image002.png)
3D Bioprinting Strategy
Advantages
Disadvantages
Resolution
Cell Density
Clogging Possibility
Applications
References
Extrusion based bioprinting
-Suitable for multi-material bioprinting applications
-Capable of printing with high cell density
-Easy-to-use bioprinting process-Slow printing speed
-Lower resolution
-Reduced cell viability-Low to medium dependent on setup of bioprinter
-High
-Yes
-Hepatocellular carcinoma model
-Colorectal cancer model
-Blood vessels[25,26,27,28]
Laser-assisted bioprinting
- Optimal cell survival
-Versatile bioink compatibility-Lower cell density
-Complicated setup and system
-Expensive cost-High (<500 nm)
-Low
-No
-Pancreatic cell network
-Glioblastoma tumor model
-Epithelium mimicking structures
-Voluminious bone tissue[29,30,31,32]
Inkjet- bioprinting
-Cell survival excellence
-Cost effective
-Precision printing
-User friendly-Restricted by bioink viscosity
-Frequent nozzle blockages
-Inconsistent drop sizes-High (>50 µm)
-Low
-Yes
-Microvascular tissue
-Mille-Feuille-like 3D structure
-Skin
-Blood vessels[33,34,35,36]
Magnetic bioprinting
-Scalable production
-Budget friendly-Constrained by available
-bioink materials-High
-High
-No
-Prostate tumor
-Adipose tissue
-Lung
-Aortic valve[37,38,39]
Coaxial bioprinting
-Microchannel integration
-Customisable tissue properties-Slow printing rate
-Reduced cell viability-Low to medium depends on bioprinter
-High
-Yes
-Microvasular tissue
-Liver sinusoid[40,41]
Acostic bioprinting
-High cell density printing
-Constrained by bioink properties
-High (5–300 µm)
-High
-No
-Colorectal cancer-cancer
[42,43]
![Figure 3: <p>Schematic figures of 3D bioprinting modalities (<strong>A</strong>) Laser-assisted bioprinting [<a href="#ref46">46</a>], (<strong>B</strong>) Inkjet-based bioprinting [<a href="#ref47">47</a>] (<strong>C</strong>) Coaxial bioprinting [<a href="#ref24">24</a>]. Created using MS Paint.</p>](/uploads/source/articles/biomaterials-connect/2025/volume2/20250015/image003.png)
![Figure 4: <p>Bioprinting technology- digital light projection (DLP) bioprinting [<a href="#ref59">59</a>]. Created using MS Paint.</p>](/uploads/source/articles/biomaterials-connect/2025/volume2/20250015/image004.png)
This review underscores the significant progress in 3D bioprinting and the development of biomaterial inks for creating spheroids, tissues, and complex organ systems. Advances in bioink design, with tailored compositions and biological functionalities, have enhanced the ability to imitate natural tissue environments and model both healthy and diseased states. Technologies like organ-on-a-chip, which integrate multiple organs into a single platform, allow for the simulation of complex biological processes, such as drug metabolism, beyond the scope of single-organ models. These breakthroughs provide powerful tools for investigating disease mechanisms, optimizing drug treatments, and enabling personalized medicine. Although challenges remain, such as achieving effective vascularization and balancing bio-ink properties, ongoing innovations in materials science and bio-fabrication hold great promise for advancing tissue engineering and realizing patient-specific regenerative therapies.
| 2D | Two-Dimensional |
| 3D | Three-Dimensional |
| PMMA | Poly (methyl methacrylate) |
| CaP | Calcium Phosphate |
| FRESH | Freeform Reversible Embedding of Suspended Hydrogels |
| VEGF | Vascular Endothelial Growth Factor |
| PCL | Polycaprolactone |
| AI | Artificial Intelligence |
| dECM | Decellularized Extracellular Matrix |
| PF-127 | Pluronic F-127 (a type of polymeric surfactant) |
| OOC | Organ-on-Chip |
| RGD | Arginine-Glycine-Aspartate (a peptide sequence) |
| MMP | Matrix Metalloproteinase (a family of proteases) |
| AM | Additive Manufacturing |
| HA | Hyaluronic Acid |
| FDA | Food and Drug Administration |
| ECM | Extracellular matrix |
| co-PLGA | Poly Lactic-co-Glycolic Acid |
| PVC | Poly Vinyl Chloride |
| Co-Cr | Cobalt-Chromium |
| GeIMA | Gelatin-Methacryloyl |
| PLGA | Poly (lactic-co-glycolic acid) |
| PLA | Polylactic Acid |
| CAD | Computer Aided-Design |
Aanchal Siwach and Shikha Chaudhary performed introductions and techniques. Ayushi Sharma performed the main body, Vishakha, and Shraddha performed challenges and conclusion. Eliza Chakraborty provided the fundamental idea, overall supervision and feedback to help in writing an effective manuscript. All authors have read and approved the manuscript.
Not applicable.
The authors declare that they have no conflict of interest.
DST-FIST Grant Sponsored by the Department of Science & Technology (DST), Government of India. Reference No. SR/FST/COLLEGE-490/2018 for “FIST Program-2018” (TPN-2014).
On behalf of her position, Professor Eliza Chakraborty of the Department of Biotechnology, Head of the Department of DST-Fist Center (Sponsored by Ministry of Science & Technology, Govt of India), MIET, Meerut, would like to thank the Government of India’s DST-Fist Center for the technological assistance.
[1] Keane, T.J.; Badylak, S.F. Biomaterials for Tissue Engineering Applications. Seminars in Pediatric Surgery Volume 23; WB Saunders: Philadelphia, PA, USA, 2014; 112–118. . [CrossRef]
[2] Tsouknidas, A. Advancements in Biomaterials for Bioengineering and Biotechnology. Int. J. Mol. Sci. 2024, 25. [CrossRef]
[3] Tripathi, S.; Mandal, S.S.; Bauri, S.; Maiti, P. 3D Bioprinting and Its Innovative Approach for Biomedical Applications. MedComm 2020, 4, e194. [CrossRef]
[4] Ghosh, S.; Yi, H.-G. A Review on Bioinks and their Application in Plant Bioprinting. Int. J. Bioprint. 2022, 8, 172–204. [CrossRef]
[5] Tibbitt, M.W.; Anseth, K.S. Hydrogels as Extracellular Matrix Mimics for 3D Cell Culture. Biotechnol. Bioeng. 2009, 103, 655–663. [CrossRef]
[6] Fang, Z.; Wu, M.C.; Lim, K.S.; Ju, L.A. Integrating Microfluidics, Hydrogels, and 3D Bioprinting for Personalized Vessel-On-A-Chip Platforms. Biomater. Sci. 2025, 13, 1131–1160. [CrossRef]
[7] Lee, W.; Lee, V.; Polio, S.; Keegan, P.; Lee, J.-H.; Fischer, K.; Park, J.-K.; Yoo, S.-S. On-Demand 3D Freeform Fabrication of Multilayered Hydrogel Scaffold with Fluidic Channels. Biotechnol. Bioeng. 2010, 105, 1178–1186. [CrossRef]
[8] Chen, Y.; Kawazoe, N.; Chen, G. Preparation of Dexamethasone-Loaded Biphasic Calcium Phosphate Nanoparticles/Collagen Porous Composite Scaffolds for Bone Tissue Engineering. Acta Biomater. 2018, 67, 341–353. [CrossRef]
[9] Murthy, H.; Mathew, D.; Nivedhidha, R.; Jose, A.; Sijo, D.A. Biomaterials and their Application. Int. J. Res. Publ. Rev. 2023, 4, 1177–1183.
[10] Capella-Monsonís, H.; Crum, R.J.; Hussey, G.S.; Badylak, S.F. Advances, Challenges, and Future Directions in the Clinical Translation of ECM Biomaterials for Regenerative Medicine Applications. Adv. Drug Deliv. Rev. 2024, 211, 115347. [CrossRef]
[11] Nii, T.; Katayama, Y. Biomaterial-Assisted Regenerative Medicine. Int. J. Mol. Sci. 2021, 22. [CrossRef]
[12] Gopinathan, J.; Noh, I. Recent Trends in Bioinks for 3D Printing. Biomater. Res. 2018, 22. [CrossRef]
[13] Guvendiren, M.; Molde, J.; Soares, R.M.; Kohn, J. Designing Biomaterials for 3D Printing. ACS Biomater. Sci. Eng. 2016, 2, 1679–1693. [CrossRef]
[14] Jammalamadaka, U.; Tappa, K. Recent Advances in Biomaterials for 3D Printing and Tissue Engineering. J. Funct. Biomater. 2018, 9. [CrossRef]
[15] Chai, H.N.; Wu, B.M. Recent Advances in 3D Printing of Biomaterials. J. Biol. Eng. 2015, 9. [CrossRef]
[16] Gogoi, D.; Kumar, M.; Singh, J. A Comprehensive Review on Hydrogel-Based Bio-Ink Development for Tissue Engineering Scaffolds Using 3D Printing. Ann. 3D Print. Med. 2024, 15, 100159. [CrossRef]
[17] Taneja, H.; Salodkar, S.M.; Parmar, A.S.; Chaudhary, S. Hydrogel Based 3D Printing: Bio Ink for Tissue Engineering. J. Mol. Liq. 2022, 367, 120390. [CrossRef]
[18] Chaudhary, S.; Chakraborty, E. Hydrogel Based Tissue Engineering and Its Future Applications in Personalized Disease Modeling and Regenerative Therapy. Beni-Suef Univ. J. Basic. Appl. Sci. 2022, 11, 1–15. [CrossRef]
[19] Shin, H.; Jo, S.; Mikos, A.G. Biomimetic Materials for Tissue Engineering. Biomaterials 2003, 24, 4353–4364. [CrossRef]
[20] Bose, S.; Bandyopadhyay, A. Introduction to Biomaterials. Characterization of Biomaterials ; Academic Press: New York, NY, USA, 2013; 1–9. https://www.cityu.edu.hk/phy/appkchu/Publications/2013/13.42.pdf.
[21] Kulinets, I. Biomaterials and Their Applications in Medicine. Regulatory Affairs for Biomaterials and Medical Devices ; Woodhead Publishing: Cambridge, UK, 2015; 1–10. . [CrossRef]
[22] You, S.; Li, J.; Zhu, W.; Yu, C.; Mei, D.; Chen, S. Nanoscale 3D Printing of Hydrogels for Cellular Tissue Engineering. J. Mater. Chem. B 2018, 6, 2187–2197. [CrossRef]
[23] Papaioannou, T.G.; Manolesou, D.; Dimakakos, E.; Tsoucalas, G.; Vavuranakis, M.; Tousoulis, D. 3D Bioprinting Methods and Techniques: Applications on Artificial Blood Vessel Fabrication. Acta Cardiol. Sin. 2019, 35, 284–289. [CrossRef]
[24] Zhuang, X.; Deng, G.; Wu, X.; Xie, J.; Li, D.; Peng, S.; Tang, D.; Zhou, G. Recent Advances of Three-Dimensional Bioprinting Technology in Hepato-Pancreato-Biliary Cancer Models. Front. Oncol. 2023, 13. [CrossRef] [PubMed]
[25] Günay, M.; Meral, T. Biomedical Applications with Multiscale Structures Produced by Additive Manufacturing. J. Mol. Eng. Mater. 2025, 13, 2430004. [CrossRef]
[26] Sun, L.; Yang, H.; Wang, Y.; Zhang, X.; Jin, B.; Xie, F.; Jin, Y.; Pang, Y.; Zhao, H.; Lu, X.; et al. Application of a 3D Bioprinted Hepatocellular Carcinoma Cell Model in Antitumor Drug Research. Front. Oncol. 2020, 10. [CrossRef]
[27] Li, Y.; Zhang, T.; Pang, Y.; Li, L.; Chen, Z.N.; Sun, W. 3D Bioprinting of Hepatoma Cells and Application with Microfluidics for Pharmacodynamic Test of Metuzumab. Biofabrication 2019, 11. [CrossRef]
[28] Sbirkov, Y.; Molander, D.; Milet, C.; Bodurov, I.; Atanasov, B.; Penkov, R.; Belev, N.; Forraz, N.; McGuckin, C.; Sarafian, V. A Colo-Rectal Cancer 3D Bioprinting Workflow as a Platform for Disease Modeling and Chemotherapeutic Screening. Front. Bioeng. Biotechnol. 2021, 9. [CrossRef]
[29] Zhang, Y.; Yu, Y.; Akkouch, A.; Dababneh, A.; Dolati, F.; Ozbolat, I.T. In Vitro Study of Directly Bioprinted Perfusable Vasculature Conduits. Biomater. Sci. 2014, 3, 134–143. [CrossRef]
[30] A Salg, G.; Poisel, E.; Neulinger-Munoz, M.; Gerhardus, J.; Cebulla, D.; Bludszuweit-Philipp, C.; Vieira, V.; Nickel, F.; Herr, I.; Blaeser, A.; et al. Toward 3D-Bioprinting of an Endocrine Pancreas: A Building-Block Concept for Bioartificial Insulin-Secreting Tissue. J. Tissue Eng. 2022, 13. [CrossRef] [PubMed]
[31] Chirivì, M.; Bearzi, C.; Rosa, P.; Miglietta, S.; Petronella, F.; De Falco, E.; Calogero, A.; Pani, R.; Petrozza, V.; Perotto, G.; et al. Biomimetic Keratin-Coated Gold Nanoparticles for Photo-Thermal Therapy in a 3d Bioprinted Glioblastoma Tumor Model. Int. J. Mol. Sci. 2022, 23. [CrossRef]
[32] Sorkio, A.; Koch, L.; Koivusalo, L.; Deiwick, A.; Miettinen, S.; Chichkov, B.; Skottman, H. Human Stem Cell Based Corneal Tissue Mimicking Structures Using Laser-Assisted 3D Bioprinting and Functional Bioinks. Biomaterials 2018, 171, 57–71. [CrossRef]
[33] Kawecki, F.; Clafshenkel, W.P.; A Auger, F.; Bourget, J.-M.; Fradette, J.; Devillard, R. Self-assembled Human Osseous Cell Sheets as Living Biopapers for the Laser-Assisted Bioprinting of Human Endothelial Cells. Biofabrication 2018, 10. [CrossRef]
[34] Solis, L.H.; Ayala, Y.; Portillo, S.; Varela-Ramirez, A.; Aguilera, R.J.; Boland, T. Thermal Inkjet Bioprinting Triggers the Activation of the VEGF Pathway in Human Microvascular Endothelial Cells in vitro. Biofabrication 2019, 11. [CrossRef] [PubMed]
[35] Takagi, D.; Lin, W.; Matsumoto, T.; Yaginuma, H.; Hemmi, N.; Hatada, S.; Seo, M. High-Precision Three-Dimensional Inkjet Technology for Live Cell Bioprinting. Int. J. Bioprint. 2019, 5, 27–38. [CrossRef]
[36] Koch, L.; Deiwick, A.; Schlie, S.; Michael, S.; Gruene, M.; Coger, V.; Zychlinski, D.; Schambach, A.; Reimers, K.; Vogt, P.M.; et al. Skin Tissue Generation by Laser Cell Printing. Biotechnol. Bioeng. 2012, 109, 1855–1863. [CrossRef] [PubMed]
[37] Xu, C.; Chai, W.; Huang, Y.; Markwald, R.R. Scaffold-Free Inkjet Printing of Three-Dimensional Zigzag Cellular Tubes. Biotechnol. Bioeng. 2012, 109, 3152–3160. [CrossRef] [PubMed]
[38] Souza, A.G.; Silva, I.B.B.; Campos-Fernandez, E.; Barcelos, L.S.; Souza, J.B.; Marangoni, K.; Goulart, L.R.; Alonso-Goulart, V. Comparative Assay of 2D and 3D Cell Culture Models: Proliferation, Gene Expression and Anticancer Drug Response. Curr. Pharm. Des. 2018, 24, 1689–1694. [CrossRef]
[39] Daquinag, A.C.; Souza, G.R.; Kolonin, M.G. Adipose Tissue Engineering in Three-Dimensional Levitation Tissue Culture System Based on Magnetic Nanoparticles. Tissue Eng. Part C Methods 2013, 19, 336–344. [CrossRef]
[40] Tseng, H.; Balaoing, L.R.; Grigoryan, B.; Raphael, R.M.; Killian, T.; Souza, G.R.; Grande-Allen, K.J. A Three-Dimensional Co-Culture Model of the Aortic Valve Using Magnetic Levitation. Acta Biomater. 2014, 10, 173–182. [CrossRef]
[41] Lian, L.; Zhou, C.; Tang, G.; Xie, M.; Wang, Z.; Luo, Z.; Japo, J.; Wang, D.; Zhou, J.; Wang, M.; et al. Uniaxial and Coaxial Vertical Embedded Extrusion Bioprinting. Adv. Health Mater. 2021, 11, 2102411. [CrossRef]
[42] Taymour, R.; Chicaiza-Cabezas, N.A.; Gelinsky, M.; Lode, A. Core–Shell Bioprinting of Vascularized in vitro Liver Sinusoid Models. Biofabrication 2022, 14. [CrossRef]
[43] Arabpour, Z.; Abedi, F.; Salehi, M.; Baharnoori, S.M.; Soleimani, M.; Djalilian, A.R. Hydrogel-Based Skin Regeneration. Int. J. Mol. Sci. 2024, 25. [CrossRef]
[44] Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D Bioprinting for Biomedical Devices and Tissue Engineering: A Review of Recent Trends and Advances. Bioact. Mater. 2018, 3, 144–156. [CrossRef] [PubMed]
[45] Hospodiuk, M.; Moncal, K.K.; Dey, M.; Ozbolat, I.T. Extrusion-Based Biofabrication in Tissue Engineering and Regenerative Medicine. 3D Printing and Biofabrication ; Springer: Cham, Switzerland, 2016; 1–27. . [CrossRef]
[46] Moroni, L.; Boland, T.; Burdick, J.A.; De Maria, C.; Derby, B.; Forgacs, G.; Groll, J.; Li, Q.; Malda, J.; Mironov, V.A.; et al. Biofabrication: A Guide to Technology and Terminology. Trends Biotechnol. 2018, 36, 384–402. [CrossRef]
[47] Huang, Y.; Zhang, X.F.; Gao, G.; Yonezawa, T.; Cui, X. 3D Bioprinting and the Current Applications in Tissue Engineering. Biotechnol. J. 2017, 12. [CrossRef] [PubMed]
[48] Cui, X.; Boland, T.; D.D’Lima, D.; Lotz, M.K. Thermal Inkjet Printing in Tissue Engineering and Regenerative Medicine. Recent. Pat. Drug Deliv. Formul. 2012, 6, 149–155. [CrossRef] [PubMed]
[49] Fernandez-Vega, V.; Hou, S.; Plenker, D.; Tiriac, H.; Baillargeon, P.; Shumate, J.; Scampavia, L.; Seldin, J.; Souza, G.R.; Tuveson, D.A.; et al. Lead Identification Using 3D Models of Pancreatic Cancer. SLAS Discov. Adv. Sci. Drug Discov. 2022, 27, 159–166. [CrossRef]
[50] Gao, Q.; He, Y.; Fu, J.Z.; Liu, A.; Ma, L. Coaxial Nozzle-Assisted 3D Bioprinting with Built-in Microchannels for Nutrients Delivery. Biomaterials 2015, 61, 203–215. [CrossRef]
[51] Böttcher, B.; Pflieger, A.; Schumacher, J.; Jungnickel, B.; Feller, K.-H. 3D Bioprinting of Prevascularized Full-Thickness Gelatin-Alginate Structures with Embedded Co-Cultures. Bioengineering 2022, 9. [CrossRef]
[52] Ozbolat, I.T.; Hospodiuk, M. Current Advances and Future Perspectives in Extrusion-Based Bioprinting. Biomaterials 2016, 76, 321–343. [CrossRef]
[53] Chen, K.; Jiang, E.; Wei, X.; Xia, Y.; Wu, Z.; Gong, Z.; Shang, Z.; Guo, S. The Acoustic Droplet Printing of Functional Tumor Microenvironments. Lab. Chip 2021, 21, 1604–1612. [CrossRef]
[54] Haisler, W.L.; Timm, D.M.; A Gage, J.; Tseng, H.; Killian, T.C.; Souza, G.R. Three-Dimensional Cell Culturing by Magnetic Levitation. Nat. Protoc. 2013, 8, 1940–1949. [CrossRef]
[55] Tseng, H.; Gage, J.A.; Raphael, R.M.; Moore, R.H.; Killian, T.C.; Grande-Allen, K.J.; Souza, G.R. Assembly of a Three-Dimensional Multitype Bronchiole Coculture Model Using Magnetic Levitation. Tissue Eng. Part C Methods 2013, 19, 665–675. [CrossRef] [PubMed]
[56] Kadry, H.; Wadnap, S.; Xu, C.; Ahsan, F. Digital light processing (DLP) 3D-Printing Technology and Photoreactive Polymers in Fabrication of Modified-Release Tablets. Eur. J. Pharm. Sci. 2019, 135, 60–67. [CrossRef]
[57] Hosseinabadi, H.G.; Dogan, E.; Miri, A.K.; Ionov, L. Digital Light Processing Bioprinting Advances for Microtissue Models. ACS Biomater. Sci. Eng. 2022, 8, 1381–1395. [CrossRef]
[58] Cecen, B.; Karavasili, C.; Nazir, M.; Bhusal, A.; Dogan, E.; Shahriyari, F.; Tamburaci, S.; Buyukoz, M.; Kozaci, L.D.; Miri, A.K. Multi-Organs-on-Chips for Testing Small-Molecule Drugs: Challenges and Perspectives. Pharmaceutics 2021, 13. [CrossRef]
[59] Sultan, T.; Lee, O.J.; Lee, J.S.; Park, C.H. Three-Dimensional Digital Light-Processing Bioprinting Using Silk Fibroin-Based Bio-Ink: Recent Advancements in Biomedical Applications. Biomedicines 2022, 10. [CrossRef] [PubMed]
[60] Sasmal, P.; Datta, P.; Wu, Y.; Ozbolat, I.T. 3D Bioprinting for Modelling Vasculature. Microphysiological Syst. 2018, 1, 1. [CrossRef] [PubMed]
[61] Zhu, Y.; Stark, C.J.; Madira, S.; Ethiraj, S.; Venkatesh, A.; Anilkumar, S.; Jung, J.; Lee, S.; Wu, C.A.; Walsh, S.K.; et al. Three-Dimensional Bioprinting with Alginate by Freeform Reversible Embedding of Suspended Hydrogels with Tunable Physical Properties and Cell Proliferation. Bioengineering 2022, 9. [CrossRef]
[62] Bakirci, E.; Adib, A.A.; Ashraf, S.F.; Feinberg, A.W. Advancing Extrusion-Based Embedded 3D Bioprinting via Scientific, Engineering, and Process Innovations. Biofabrication 2025, 17. [CrossRef]
[63] Khare, V.; Parihar, A.; Khan, R.; Chandel, A.S. Biomaterials: An Overview for Future Utility in the Biomedical Domain. Smart Ways of Biomaterial Designing Synthesis and Characterization ; CRC Press: Boca Raton, FL, USA, 2025; 1–20. . [CrossRef]
[64] Shusteff, M.; Browar, A.E.M.; Kelly, B.E.; Henriksson, J.; Weisgraber, T.H.; Panas, R.M.; Fang, N.X.; Spadaccini, C.M. One-Step Volumetric Additive Manufacturing of Complex Polymer Structures. Sci. Adv. 2017, 3, eaao5496. [CrossRef]
[65] Kelly, B.E.; Bhattacharya, I.; Heidari, H.; Shusteff, M.; Spadaccini, C.M.; Taylor, H.K. Volumetric Additive Manufacturing via Tomographic Reconstruction. Science 2019, 363, 1075–1079. [CrossRef]
[66] Rodríguez-Pombo, L.; Xu, X.; Seijo-Rabina, A.; Ong, J.J.; Alvarez-Lorenzo, C.; Rial, C.; Nieto, D.; Gaisford, S.; Basit, A.W.; Goyanes, A. Volumetric 3D Printing for Rapid Production of Medicines. Addit. Manuf. 2022, 52, 102673. [CrossRef]
[67] Heydari, Z.; Moeinvaziri, F.; Agarwal, T.; Pooyan, P.; Shpichka, A.; Maiti, T.K.; Timashev, P.; Baharvand, H.; Vosough, M. Organoids: A Novel Modality in Disease Modeling. Bio-Des. Manuf. 2021, 4, 689–716. [CrossRef]
[68] Gunti, S.; Hoke, A.T.K.; Vu, K.; London, N.R. Organoid and Spheroid Tumor Models: Techniques and Applications. Cancers 2021, 13. [CrossRef] [PubMed]
[69] Moradi, N.; Vahdat, S. An Overview of Three-Dimensional Culture Systems for Recapitulation Human Organs in Research: Advances and Applications. Pathobiol. Res. 2024, 27, 7–16.
[70] Zhang, S.-C.; Wernig, M.; Duncan, I.D.; Brüstle, O.; Thomson, J.A. In vitro Differentiation of Transplantable Neural Precursors from Human Embryonic Stem Cells. Nat. Biotechnol. 2001, 19, 1129–1133. [CrossRef]
[71] Lewis-Israeli, Y.R.; Wasserman, A.H.; Gabalski, M.A.; Volmert, B.D.; Ming, Y.; Ball, K.A.; Yang, W.; Zou, J.; Ni, G.; Pajares, N.; et al. Self-Assembling Human Heart Organoids for the Modeling of Cardiac Development and Congenital Heart Disease. Nat. Commun. 2021, 12, 1–16. [CrossRef]
[72] Maji, S.; Lee, H. Engineering Hydrogels for the Development of Three-Dimensional in vitro Models. Int. J. Mol. Sci. 2022, 23. [CrossRef]
[73] Suhag, D.; Kaushik, S.; Taxak, V.B. Challenges and Future Directions. Handbook of Biomaterials for Medical Applications, Volume 1: Fundamentals ; Springer Nature Singapore: Singapore, 2024; 329–355. . [CrossRef]
[74] Mao, H.; Yang, L.; Zhu, H.; Wu, L.; Ji, P.; Yang, J.; Gu, Z. Recent Advances and Challenges in Mate-Rials for 3D Bioprinting. Progress. Nat. Sci. Mater. Int. 2020, 30, 618–634. [CrossRef]
[75] Martin, R.; Joung, D. The Promise and Challenges of Bioprinting in Tissue Engineering. Micromachines 2024, 15. [CrossRef]
[76] Bayer, E.; Gottardi, R.; Fedorchak, M.; Little, S. The Scope and Sequence of Growth Factor Delivery for Vascularized Bone Tissue Regeneration. J. Control. Release 2015, 219, 129–140. [CrossRef]
[77] Liu, Y.; Chan, J.K.; Teoh, S.H. Review of Vascularised Bone Tissue-Engineering Strategies with a Focus on Co-Culture Systems. J. Tissue Eng. Regen. Med. 2015, 9, 85–105. [CrossRef] [PubMed]
[78] Lee, H.; Kim, J.; Choi, Y.; Cho, D.-W. Application of Gelatin Bioinks and Cell-Printing Technology to Enhance Cell Delivery Capability for 3D Liver Fibrosis-on-a-Chip Development. ACS Biomater. Sci. Eng. 2020, 6, 2469–2477. [CrossRef]
[79] Bhatia, S.N.; Ingber, D.E. Microfluidic Organs-on-Chips. Nat. Biotechnol. 2014, 32, 760–772. [CrossRef] [PubMed]
[80] Lee, H.; Chae, S.; Kim, J.Y.; Han, W.; Kim, J.; Choi, Y.; Cho, D.-W. Cell-Printed 3D Liver-on-a-Chip Pos-Sessing a Liver Microenvironment and Biliary System. Biofabrication. 2019, 11. [CrossRef] [PubMed]
[81] Choi, Y.-J.; Park, H.; Ha, D.-H.; Yun, H.-S.; Yi, H.-G.; Lee, H. 3D Bioprinting of in vitro Models Using Hydrogel-Based Bioinks. Polymers 2021, 13. [CrossRef]
[82] Panwar, A.; Tan, L.P. Current Status of Bioinks for Micro-Extrusion-Based 3D Bioprinting. Molecules 2016, 21. [CrossRef]
[83] Jang, J.; Kim, T.G.; Kim, B.S.; Kim, S.-W.; Kwon, S.-M.; Cho, D.-W. Tailoring Mechanical Properties of Decellularized Extracellular Matrix Bioink by Vitamin B2-Induced Photo-Crosslinking. Acta Biomater. 2016, 33, 88–95. [CrossRef]
[84] Ouyang, L.; Highley, C.B.; Sun, W.; Burdick, J.A. A Generalizable Strategy for the 3D Bioprinting of Hydrogels from Nonviscous Photo-Crosslinkable Inks. Adv. Mater. 2016, 29, 1604983. [CrossRef]
[85] Singh, Y.P.; Bandyopadhyay, A.; Mandal, B.B. 3D Bioprinting Using Cross-Linker-Free Silk–Gelatin Bioink for Cartilage Tissue Engineering. ACS Appl. Mater. Interfaces 2019, 11, 33684–33696. [CrossRef]
[86] Cui, X.; Li, J.; Hartanto, Y.; Durham, M.; Tang, J.; Zhang, H.; Hooper, G.J.; Lim, K.S.; Woodfield, T.B.F. Advances in Extrusion 3D Bioprinting: A Focus on Multicomponent Hydrogel-Based Bioinks. Adv. Healthc. Mater. 2020, 9, 1901648. [CrossRef]
[87] Blume, C.; Swindle, E.J.; Dennison, P.; Jayasekera, N.P.; Dudley, S.; Monk, P.; Behrendt, H.; Schmidt-Weber, C.B.; Holgate, S.T.; Howarth, P.H. Barrier Responses of Human Bronchial Epithelial Cells to Grass Pollen Exposure. Eur. Respir. J. 2013, 42, 87–97. [CrossRef]
[88] Skardal, A.; Murphy, S.V.; Devarasetty, M.; Mead, I.; Kang, H.-W.; Seol, Y.-J.; Zhang, Y.S.; Shin, S.-R.; Zhao, L.; Aleman, J. Multi-Tissue Interactions in an Integrated Three-Tissue Organ-on-a-Chip Platform. Sci. Rep. 2017, 7, 1–16. [CrossRef] [PubMed]
[89] Skardal, A.; Devarasetty, M.; Forsythe, S.; Atala, A.; Soker, S. A Reductionist Metastasis-on-a-Chip Platform for in vitro Tumor Progression Modeling and Drug Screening. Biotechnol. Bioeng. 2016, 113, 2020–2032. [CrossRef]
[90] Singh, S.; Choudhury, D.; Yu, F.; Mironov, V.; Naing, M.W. In Situ Bioprinting—Bioprinting from Benchside to Bedside?. Acta Biomater. 2019, 101, 14–25. [CrossRef] [PubMed]
[91] Hakimi, N.; Cheng, R.; Leng, L.; Sotoudehfar, M.; Ba, P.Q.; Bakhtyar, N.; Amini-Nik, S.; Jeschke, M.G.; Günther, A. Handheld Skin Printer: In situ Formation of Planar Biomaterials and Tissues. Lab Chip 2018, 18, 1440–1451. [CrossRef]
[92] Li, L.; Yu, F.; Shi, J.; Shen, S.; Teng, H.; Yang, J.; Wang, X.; Jiang, Q. In situ Repair of Bone and Cartilage Defects Using 3D Scanning and 3D Printing. Sci. Rep. 2017, 7, 1–12. [CrossRef]
[93] Albanna, M.; Binder, K.W.; Murphy, S.V.; Kim, J.; Qasem, S.A.; Zhao, W.; Tan, J.; El-Amin, I.B.; Dice, D.D.; Marco, J.; et al. In situ Bioprinting of Autologous Skin Cells Accelerates Wound Healing of Extensive Excisional Full-Thickness Wounds. Sci. Rep. 2019, 9, 1–15. [CrossRef] [PubMed]
[94] Karamchand, L.; Makeiff, D.; Gao, Y.; Azyat, K.; Serpe, M.J.; Kulka, M. Biomaterial Inks and Bioinks for Fabricating 3D Biomimetic Lung Tissue: A Delicate Balancing Act between Biocompatibility and Mechanical Printability. Bioprinting 2022, 29. [CrossRef]
[95] Sakai, L.; Keene, D.; Glanville, R.; Bächinger, H. Purification and Partial Characterization of Fibrillin, a Cysteine-Rich Structural Component of Connective Tissue Microfibrils. J. Biol. Chem. 1991, 266, 14763–14770. [CrossRef]
[96] Lutolf, M.P.; Raeber, G.P.; Zisch, A.H.; Tirelli, N.; Hubbell, J.A. Cell-Responsive Synthetic Hydrogels. Adv. Mater. 2009, 15, 888–892. [CrossRef]
[97] E Jakus, A.; Rutz, A.L.; Shah, R.N. Advancing the Field of 3D Biomaterial Printing. Biomed. Mater. 2016, 11. [CrossRef] [PubMed]
[98] Maher, P.; Keatch, R.; Donnelly, K.; Mackay, R.; Paxton, J. Construction of 3D Biological Matrices Using Rapid Prototyping Technology. Rapid Prototyp. J. 2009, 15, 204–210. [CrossRef]
[99] Hoque, M.E.; San, W.Y.; Wei, F.; Li, S.; Huang, M.-H.; Vert, M.; Hutmacher, D.W. Processing of Polycapro-Lactone and Polycaprolactone-Based Copolymers into 3D Scaffolds, and Their Cellular Responses. Tissue Eng. 2009, A15, 3013–3024. [CrossRef] [PubMed]
[100] Semba, J.A.; Mieloch, A.A.; Tomaszewska, E.; Cywoniuk, P.; Rybka, J.D. Formulation and Evaluation of a Bioink Composed of Alginate, Gelatin, and Nanocellulose for Meniscal Tissue Engineering. Int. J. Bioprint. 2022, 9. [CrossRef]
[101] Park, S.A.; Lee, S.H.; Kim, W.D. Fabrication of Porous Polycaprolactone/Hydroxyapatite (PCL/HA) Blend Scaffolds Using a 3D Plotting System for Bone Tissue Engineering. Bioprocess. Biosyst. Eng. 2011, 34, 505–513. [CrossRef]
We use cookies to improve your experience on our site. By continuing to use our site, you accept our use of cookies. Learn more